43 acid and base worksheet answers
CHAPTER 8 Acids and Bases - Homework. Worksheet 1 – Grade 11 Acids + Bases Read Pgs 191 – 202. Pg 207 Q# 1 – 18 No Worksheet – Acid Deposition Read Pgs 204 - 206 Worksheet 2 – Acids + Bases Equilibrium Read Pgs 398 – 402 DNA Base Pairing Worksheet There are base pairing rules for writing complimentary DNA strands for a given strand. A pairs with T C pairs with G In RNA, A pairs with U, instead of T. Write the complimentary DNA strand for each given strand of DNA. 1. CGTAAGCGCTAATTA 2. TCTTAAATGATCGATC 3. AATGAATAGCTAGCTT 4. GGCATTCGCGATCATG 5. …
34. Boric acid frequently is used as an eyewash to treat eye infections. The pH of a 0.050 M solution of boric acid is 5.28. What is the value of the boric acid ionization constant, Ka? a. 5.25 ×10–6 d. 5.79 –4 b. 5.51 ×10–10 e. 5.33 ×10–12 c. 5.43 ×10–8 35. …

Acid and base worksheet answers
Ph and poh worksheet 2 answer keyWhat do the following values of poh indicate about sol n acidity. Download Free Solutions Acids And Bases Review Worksheet Answers Solutions Acids And Bases Review Worksheet Answers When somebody should go to the books stores, search establishment by shop, shelf by shelf, it is in reality problematic. Acids-and-Bases-Practice-Worksheets-with-Full-Solutions-ChemistNate-July-2021.pdf: File Size: 7170 kb: File Type: pdf: Download File. Step 2: Do the questions, and follow along with this video for when you get stuck: This workbook contains questions about: pH of a Strong Acid pH of a Weak Acid Read Book Strength Of Acids And Bases Worksheet Answers pH of Common Acids and Bases. The pH of a solution depends on the strength of the acid or base in the solution. Measurements of the pH of dilute solutions are therefore good indicators of the relative strengths of acids and bases. Values of the pH of 0.10 M solutions of a number of common ...
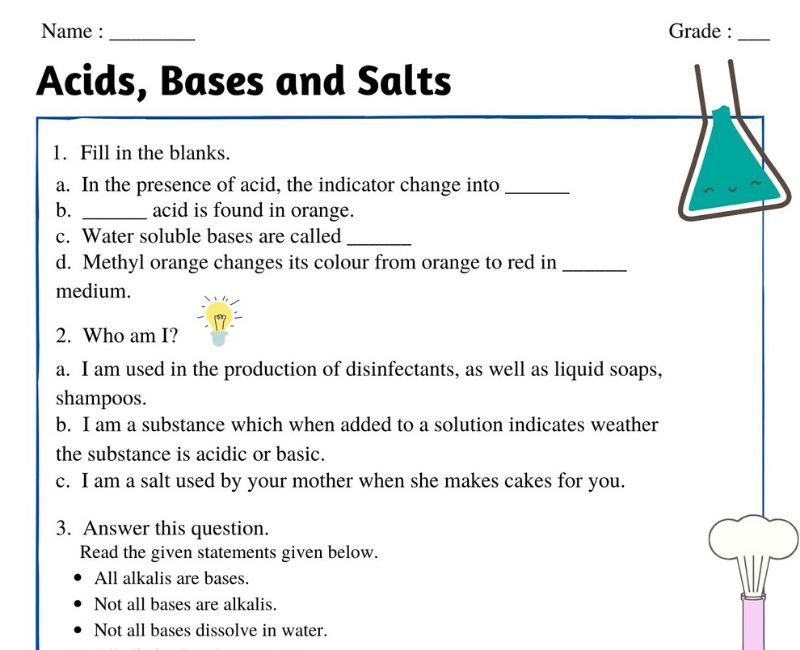
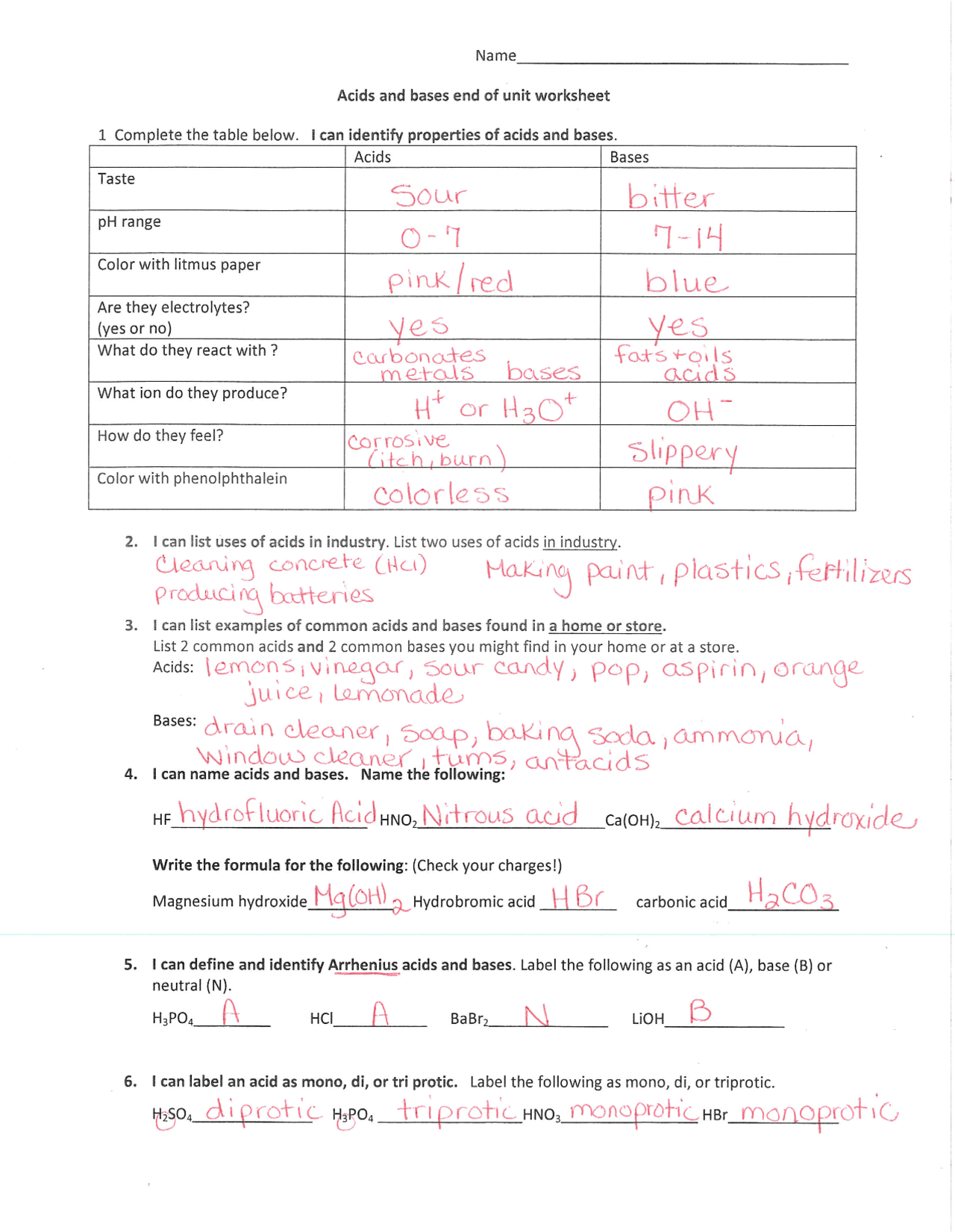
Acid and base worksheet answers. Acid-Base Review Worksheet-Accel Name: _ Per: - -10 Complete the following. Show ('U of your work! Box or circle y r answer. Identify & describe the properties of acids and bases l. Compare and contrast the following: a. Acid properties a d baÌyroperties pH ) d. Acid-base indicator and pH meter iscu (jives 14 is f. Binary acid and ternary acid Titrations worksheet W 336 Everett Community College Tutoring Center Student Support Services Program 1) It takes 83 mL of a 0.45 M NaOH solution to neutralize 235 mL of an HCl solution. What is the concentration of the HCl solution? 2) You are titrating an acid into a base to determine the concentration of the base. The Nov 07, 2021 · Definitions of Acids and Bases. We can define acids as substances that dissolve in water to produce H + ions, whereas bases are defined as substances that dissolve in water to produce OH − ions. In fact, this is only one possible set of definitions. Although the general properties of acids and bases have been known for more than a thousand years, the definitions … Identify whether the substance is an acid or a base and indicate what colour the pH indicator will turn. (a) Substance pH Value Acid or Base Methyl Orange Bromothymol Blue Litmus lemon ammonia milk (b) Substance pH Value Acid or Base Methyl Red Phenolphthalein Indigo Carmine tomato oven cleaner egg 3. Complete the following table.
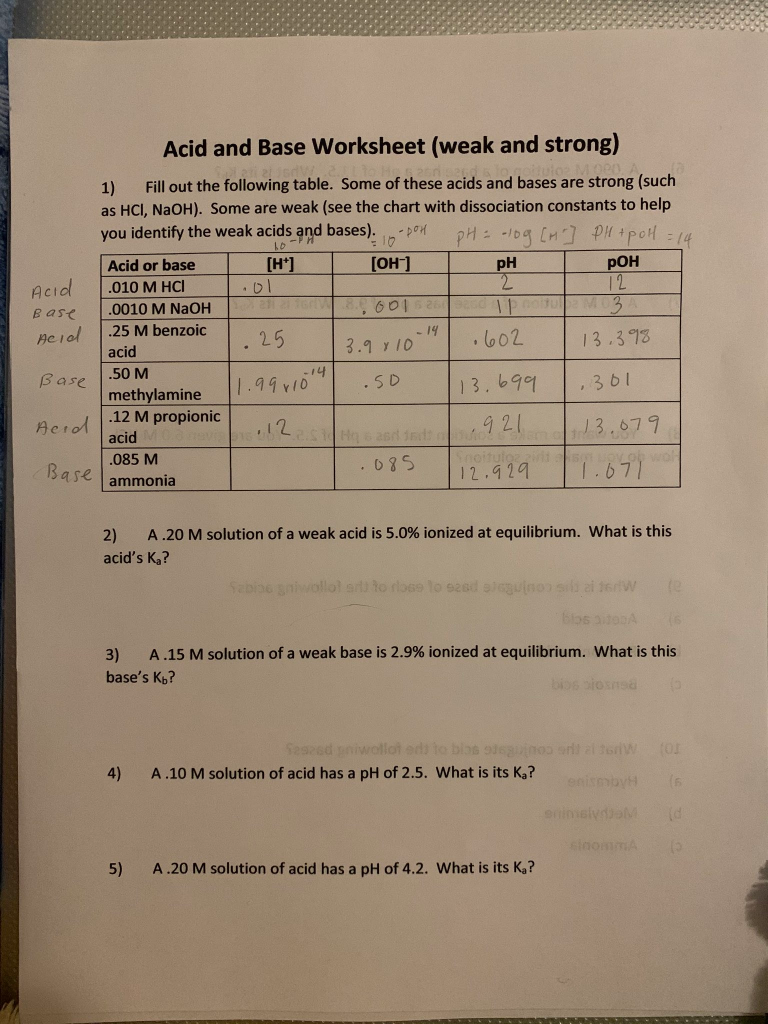
Aug 26, 2021 · Acid-Base Balance. Everyday processes like walking, the digestion of food, and the overall metabolism in your body produce a lot of acid as a byproduct. • Find a codon’s first base in the first column of the chart; stay in this row. • Find the second base in the middle of the chart, stay in this box. • Locate the third base in the far right column, this is the amino acid that matches the mRNA codon. • Warn students against using the tRNA anticodon when using the chart. In both cases identify the conjugate acid– base pairs. When lithium oxide (Li. 2. O) is dissolved in water, the solution turns basic from the reaction of the oxide ion (O. 2 –) with water. Write the reaction that occurs, and identify the conjugate acid– base pairs. Answer: O. 2– (aq) + H. 2. O(l) →OH – (aq) + OH – (aq). OH – is ... Dom pH: 0 CM PM pollera Acid or base [H] [OH-] PH POH .010 M HCl .DL I 2 I 12 .0010 M NaOH. 8. 0015201; Question: Acid and Base Worksheet (weak and strong) Acid Base Acid 1) Fill out the following table. Some of these acids and bases are strong (such as HCl, NaOH). Some are weak (see the chart with dissociation constants to help you identify ...
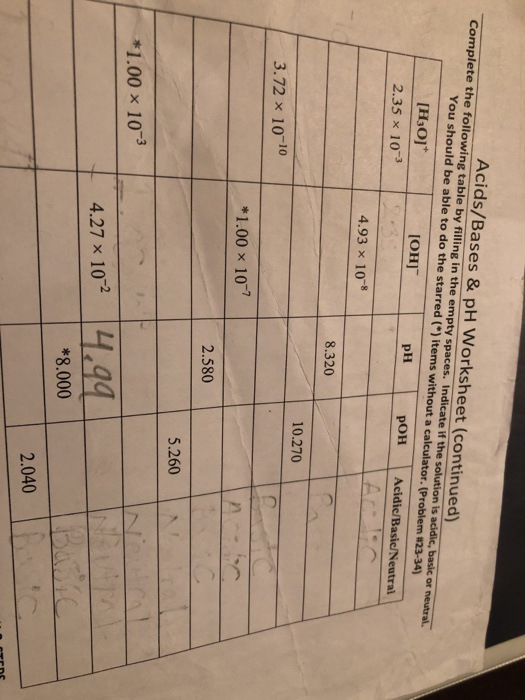
View Chapter # 3_Acid & Base_Q & A.docx from CHEMISTRY 211 at West Los Angeles College. Chapter # 3: Acid and Bases, Questions and Answers 1. What is/are the product(s) of the following acid-base Aug 25, 2021 · For acid and base dissociation, the same concepts apply, except that we use Ka or Kb instead of Kc. High values of Ka mean that the acid dissociates well and that it is a … Title: Strength Of Acids And Bases Worksheet Answers Author: pressroom.sheetz.com-2022-01-24T00:00:00+00:01 Subject: Strength Of Acids And Bases Worksheet Answers Acids & Bases Calculations Practice Worksheet Directions: Solve the followingpH calculations. Write the formula, plug numbers into formula, & give answer with correct units If the pH of a solution is 10.3, what is the [H+] concentration? -c M HC104, what is the pH? Is the solution ACIDIC, BASIC, or NEUTRAL? 2. Ifthe IH+] is 2.1 10-12 -10 3.
The simplest acid-base reactions are those of a strong acid with a strong base. Table 4 shows data for the titration of a 25.0-mL sample of 0.100 M hydrochloric acid with 0.100 M sodium hydroxide. The values of the pH measured after successive additions of small amounts of NaOH are listed in the first column of this table, and are graphed in Figure 1, in a form that is called a …
Q.17- Choose the incorrect statement out of the followings a) Deoxy hemoglobin is a weak base b) Oxyhemoglobin is a relatively strong acid c) The buffering capacity of hemoglobin is lesser than plasma protein d) The buffering capacity of Hemoglobin is due to histidine residues. Q.18- Carbonic anhydrase is present at all places except-
Read Book Strength Of Acids And Bases Worksheet Answers pH of Common Acids and Bases. The pH of a solution depends on the strength of the acid or base in the solution. Measurements of the pH of dilute solutions are therefore good indicators of the relative strengths of acids and bases. Values of the pH of 0.10 M solutions of a number of common ...
Acids-and-Bases-Practice-Worksheets-with-Full-Solutions-ChemistNate-July-2021.pdf: File Size: 7170 kb: File Type: pdf: Download File. Step 2: Do the questions, and follow along with this video for when you get stuck: This workbook contains questions about: pH of a Strong Acid pH of a Weak Acid
Ph and poh worksheet 2 answer keyWhat do the following values of poh indicate about sol n acidity. Download Free Solutions Acids And Bases Review Worksheet Answers Solutions Acids And Bases Review Worksheet Answers When somebody should go to the books stores, search establishment by shop, shelf by shelf, it is in reality problematic.

























0 Response to "43 acid and base worksheet answers"
Post a Comment