43 more average atomic mass worksheet answers
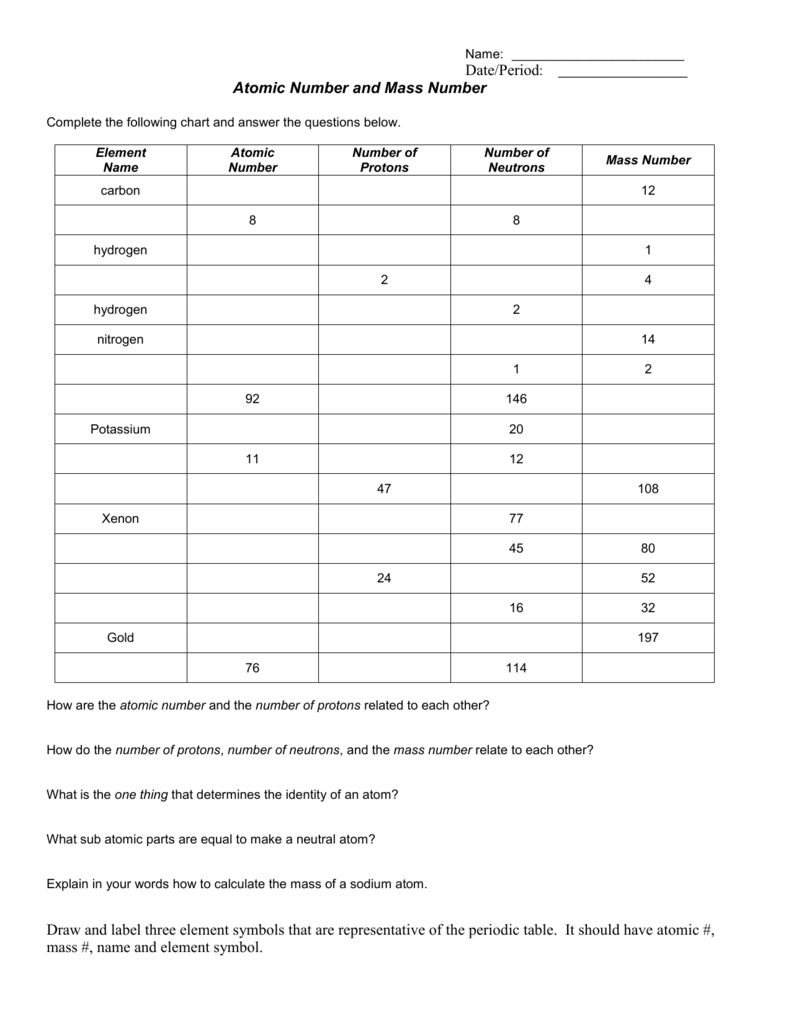
Average Atomic Mass Worksheet. by. Ms Stricklin Chemistry Corner. 4. $2.00. Word Document File. This worksheet walks students through the steps of calculating the average atomic mass of an element. Students will then practice through a couple of word problems. The number of neutrons in an isotope may be determined by subtracting the number of protons (atomic number) from the mass number. #n0 = A - Z. If working with the element, the atomic mass may be rounded to a whole number and used as a mass number. 9. An atomic nucleus contains 6 protons and 6 neutrons.
Based on the atomic mass, which isotope should be more abundant? Answer: The atomic mass of boron is 10.811; therefore, boron-11 is more abundant because the mass number is closer to the atomic mass. 8. Lithium-6 is 4% abundant and lithium-7 is 96% abundant. What is the average mass of lithium? Answer: 6.96 amu 9.

More average atomic mass worksheet answers
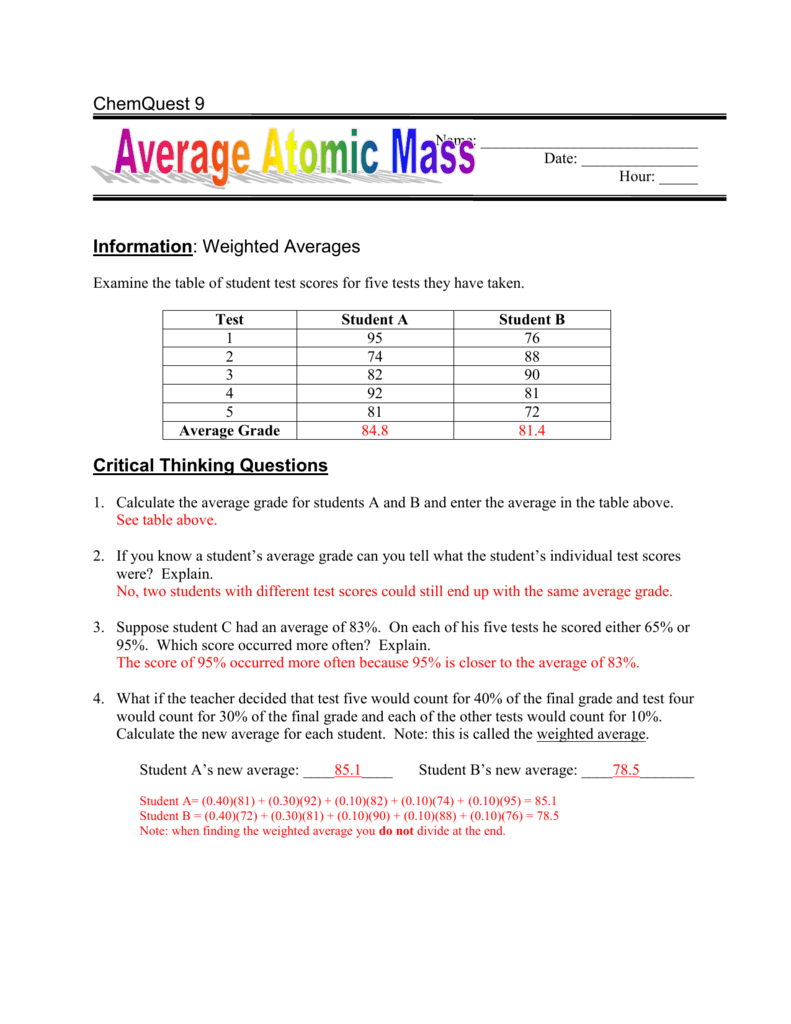
Refer to the atomic masses in the periodic table inside the front cover of this textbook. State the mass of Avogadro's number of atoms for each of the following elements: (a) Copper (c) sulfur (b) Mercury (d) helium Refer to the periodic table and state the mass for each of the following: (a) 1 atom of Au (b) 6.02 . ×. 1023 atoms of Au. Answers: 37. Calculate the average atomic mass of chlorine. 2. Copper has two isotopes. Copper-63, which has an atomic mass of 62.93 u and copper-65, which has an atomic mass of 64.93 u. In any sample of copper atoms, 69.1% will be copper-63 and 30.9% will be copper-65. Calculate the average atomic mass of naturally occurring copper. 3. One atom has 20 ... Given that the average atomic mass of the elements in the body is 6 g/mol, we get. 68,000 g = = 11,333 mol. Section 6.2: Team Learning Worksheet. 1. The term "atomic mass" refers to the mass of one atom, while the term "molar mass" refers to the mass of 1 mol of a substance (either an element or a compound).
More average atomic mass worksheet answers. Mass and weight worksheet answers. 6 x 160 960 ____. 20 Acids And Bases Practice Problems Answers Round atomic masses to the tenth of a decimal place. Worksheet mass mass problems answers. Ad Download over 20000 K-8 worksheets covering math reading social studies and more. Molar mass worksheet answer key 399370. 6 how many grams […] August 16, 2021. August 13, 2021 By. admin. Average Atomic Mass Worksheet. Encouraged to be able to my website, in this particular period I am going to teach you in relation to Average Atomic Mass Worksheet. Why not consider photograph over? can be which remarkable???. if you think therefore, I'l l show you many image yet again beneath: The afterward definitions may be helpful: Atomic cardinal is the cardinal of protons in an element. This cardinal is consistently the aforementioned for every atom of a accurate element; it is a axiological acreage of the element. Atomic accumulation is the… Read More "Calculating Average Atomic Mass Worksheet" » CHM 4, PAL - atomic mass Student name: 2 Part B: Atomic mass calculations 4) Naturally occurring lithium consists of two isotopes, Li-6 with a mass of 6.015 amu and an abundance of 7.42% and Li-7 with a mass of 7.016 amu. Start by filling out the table below and then calculate the atomic mass of lithium. Show all your work
Are all atoms of an element the same? How can you tell one isotope from another? Use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element. An average atomic mass worksheet answers pogil in the word s authentic meaning is a piece of. Atomic mass and atomic number worksheet key name of element symbol atomic number atomic mass protons neutrons electrons copper cu 29 64. Exam elaborations student exploration. Average atomic mass f 1 m 1 f 2 m 2. name: !suggested answers date: _____ ! isotopic abundance - practice problems The atomic mass for each element appearing on the periodic table represents the weighted average of masses for each individual isotope of an element. For example, the atomic mass of carbon is reported as 12.011 amu (atomic mass units). Carbon is composed primarily of two isotopes; carbon-12 and carbon-14. Calculate the average atomic mass. 6) Copper used in electric wires comes in two flavors (isotopes): 63Cu and 65Cu. 63Cu has an atomic mass of 62.9298 amu and an abundance of 69.09%. The other isotope, 65Cu, has an abundance of 30.91%. The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu.
15. Use one of the methods in Model 3 that gave the correct answer for average atomic mass to calculate the average atomic mass for oxygen. Isotope information is provided below. Show all of your work and check your answer against the mass listed on the periodic table. Isoto e Natural Abundance on Earth (0/0) Atomic Mass (am u) - 16.00 160 170 180 More Average Atomic Mass. Calculate the average atomic masses. Round all answers to two decimal places. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? Iodine is 80% 127I, 17% 126I, and 3% 128I. More Average Atomic Mass Calculate the average atomic masses. Round all answers to two decimal places. 1. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? 178.55 amu 2. 15. Use one of the methods in Model 3 that gave the correct answer for average atomic mass to calculate the average atomic mass for oxygen. Isotope information is provided below. Show all of your work and check your answer against the mass listed on the periodic table. Isotope Natural Abundance on Earth (%) Atomic Mass (amu) 160 99.76 15.9949
The atomic mass for each element listed in the periodic table is actually the weighted average mass of all of the different isotopes of the element. In the Average Atomic Mass Gizmo, use a mass spectrometer to separate an element into its isotopes. Then, calculate the average atomic mass by considering the mass and abundance of each isotope.
02 06 Average Atomic Mass Worksheet 2 Name 17 What is the average atomic mass of from CHE INORGANIC at Northside High School
Related posts: Average Atomic Mass Gizmo Worksheet Answers Show all of your work and check your answer against...; Average Atomic Mass Gizmo Activity A Answer Key Test student a student b. All helium atoms have 2... Average Atomic Mass Gizmo Assessment Answer Key 435 have a mass of 499461 amu 8379 have amass...; Average Atomic Mass Lab Gizmo Answer Key Average Atomic Mass Answer Key Vocabulary.
Isotopes and average atomic mass, as concepts, allow for the specific discussion of elements and their atoms, and this quiz/worksheet combo will help you test your understanding of these concepts.
More Average Atomic Mass. Calculate the average atomic masses. Round all answers to two decimal places. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? Iodine is 80% 127I, 17% 126I, and 3% 128I.
The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu. X — amð 7) Magnesium consists of three naturally occurring isotopes. The percent abundance of these isotopes is as follows: 24 Mg (78.70%), 25Mg (10.13%), and 26Mg(11.7%). 'The average atomic mass of the three isotopes is 24.3050 amu.
The afterward definitions may be helpful: Atomic cardinal is the cardinal of protons in an element. This cardinal is consistently the aforementioned for every atom of a accurate element; it is a axiological acreage of the element. Atomic accumulation is the… Read More "Calculating Average Atomic Mass Worksheet" »
average atomic mass worksheet answer key by online. You might not require more time to spend to go to the books creation as well as search for them. In some cases, you likewise pull off not discover the publication average atomic mass worksheet answer key that you are looking for. It will certainly squander the time.
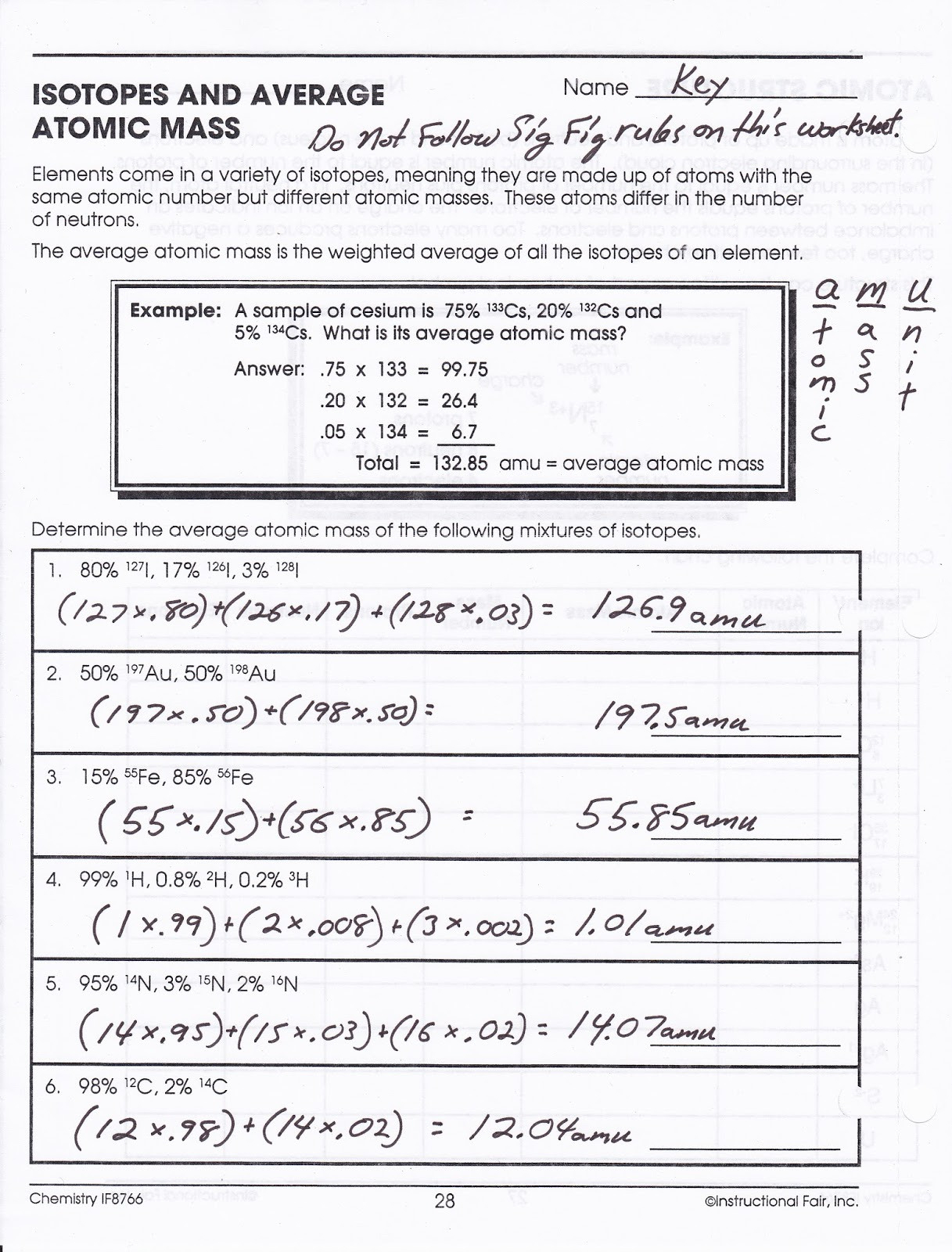
The average atomic mass is the weighted average of all the isotopes of an element. Example: A sample of cesium is 75% 133Cs, 20% 132Cs and 5% 134Cs. What is its average atomic mass? Answer: .75 x 133 = 99.75 .20 x 132 = 26.4 .05 x 134 = Total = 132.85 amu = average atomic mass Determine the average atomic mass of the following mixtures of ...
Average Atomic Mass Worksheet Posted on August 16, 2021 August 13, 2021 By admin Encouraged to be able to my website, in this particular period I am going to teach you in relation to Average Atomic Mass Worksheet.
Given that the average atomic mass of the elements in the body is 6 g/mol, we get. 68,000 g = = 11,333 mol. Section 6.2: Team Learning Worksheet. 1. The term "atomic mass" refers to the mass of one atom, while the term "molar mass" refers to the mass of 1 mol of a substance (either an element or a compound).
37. Calculate the average atomic mass of chlorine. 2. Copper has two isotopes. Copper-63, which has an atomic mass of 62.93 u and copper-65, which has an atomic mass of 64.93 u. In any sample of copper atoms, 69.1% will be copper-63 and 30.9% will be copper-65. Calculate the average atomic mass of naturally occurring copper. 3. One atom has 20 ...
Refer to the atomic masses in the periodic table inside the front cover of this textbook. State the mass of Avogadro's number of atoms for each of the following elements: (a) Copper (c) sulfur (b) Mercury (d) helium Refer to the periodic table and state the mass for each of the following: (a) 1 atom of Au (b) 6.02 . ×. 1023 atoms of Au. Answers:


































0 Response to "43 more average atomic mass worksheet answers"
Post a Comment