41 Atomic Mass And Atomic Number Worksheet
Atomic and mass number 2. • Atomic number is the number of Protons in an atom • All atoms of a particular element have the SAME number of protons (All Carbon atoms 4. Calculate the mass number of each of the following atoms. 5. O 8 16 Atomic number Mass number Symbol OxygenName Atomic number= Number... The Parts of the Periodic Table | Atomic Numbers The atomic number is the number of protons in the nucleus of an atom. In the original periodic table published by Dimitri Mendeleev in 1869, the elements were arranged according to increasing atomic mass — at that time, the nucleus had not yet been discovered, and there was no understanding at all...
PDF Microsoft Word - Atomic Mass and Atomic Number Worksheet Key Atomic Mass. 64 119 127 238 39.

Atomic mass and atomic number worksheet
Atomic number, Mass number and Isotopes - презентация онлайн What are its atomic number and mass number? 1 H 1.00797 • This atomic mass is the one number that best represents the mass of all three versions of hydrogen. • No atom of hydrogen anywhere in the universe actually has this mass. • Periodic Table Atomic Mass - Trick To Learn Atomic Mass Number How To Find The Atomic Number Of All Elements In Periodic Table ||Trick To Learn Periodic Table ||. Atomic number, atomic mass, and isotopes (article) | Khan Academy Fundamental properties of atoms including atomic number and atomic mass. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons.
Atomic mass and atomic number worksheet. Atomic Number and Mass Number When considering atomic mass, it is customary to ignore the mass of any electrons and calculate the atom's mass based on the number of protons and neutrons alone. Electrons contribute greatly to the atom's charge, as each electron has a negative charge equal to the positive charge of a proton. Standard Atomic Weights | Commission on Isotopic Abundances and... Standard atomic weights are CIAAW recommended values for atomic weights applicable to all normal materials. Since 1902, the Commission regularly publishes critical evaluation of atomic weights of elements and below is the most recent definitive table of the standard atomic weights. PDF Basic Atomic Structure Worksheet The 3 particles of the atom The atomic number gives the "identity" of an element as well as its location on the periodic table. No two different elements will have the. atomic number. of an element is the average mass of an element's naturally Occurring atom, or _ of each isotope. Atomic Mass and Atomic Number Worksheet... | CK-12 Foundation Atomic Number. Explains the arrangement of the periodic table and the relationship between subatomic particles. % Progress. A worksheet providing practice in using the periodic table to fill out information on various elements. Difficulty Level.
3.4: Atomic Mass and Atomic Number - Chemistry LibreTexts Knowing the mass number and the atomic number of an atom allows you to determine the number of neutrons present in that atom by subtraction. The composition of any atom can be illustrated with a shorthand notation called A/Z format. Both the atomic number and mass are written to the left of the... PDF Isotopes: atoms with identical atomic numbers but different mass... · One atomic mass unit (amu): the mass exactly equal to one-twelfth the mass of one carbon-12 atom that has six protons and six neutrons. Mass number (number of protons plus neutrons). Carbon Isotopes. Atomic Number and Mass Number | Difference Between Atomic... The atomic number is always calculated by the number of protons. For instance, If an atom has 1 proton then the atomic number is 1 and the element is hydrogen. Normally, atomic mass and mass numbers are two different terms and may differ slightly. In most cases, they are not the same. PDF Atomic structure This means that atoms of different elements atoms must have different masses. Mass Number: The number of protons and neutrons in the nucleus of an atom. • Atomic number - the number of protons = 3 • Atoms are neutral - 3 protons and 3 electrons. •
Atomic number and mass number - Atomic structure - AQA - GCSE... Atomic structure. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Atoms of different elements usually have different mass numbers, but they can be the same. For example, the mass number of argon atoms and calcium atoms can both be 40. Periodic Table with Atomic Mass | Science Notes and Projects The atomic mass is the average number of protons and neutrons in atoms of a chemical elements, allowing for the natural abundances of the If you choose "Fit to Paper" and your printer will scale the table down without any loss of readability. Periodic Table with Atomic Mass - Black and White Edition. Atomic Mass Formula The atomic mass of an element is equal to the weighted average of the isotopes for that element. The number of protons determines the identity of the atom, and isotopes have identical atomic numbers, so the atoms are of the same element. 3 Ways to Calculate Atomic Mass - wikiHow The atomic mass is the number of grams of the element in one mole of atoms of the element. This is a very useful property when it comes to practical calculations, as it allows easy Relative atomic masses, as listed on the periodic table, are used to calculate molar masses for atoms and molecules.
Atomic number and atomic mass worksheet | Teaching Resources Subject: Chemistry. Age range: 14-16. Resource type: Worksheet/Activity. (no rating) 0 reviews. Megmcb.
Atomic mass - Wikipedia The atomic mass (ma or m) is the mass of an atom. Although the SI unit of mass is the kilogram (symbol: kg), atomic mass is often expressed in the non-SI unit atomic mass unit (amu) or unified mass (u) or dalton (symbol: Da), where 1 amu or 1 u or 1 Da is defined as.
Atomic Number And Atomic Mass Number : Definition, Examples... Atomic Mass and Atomic Mass number are different terms and should be used carefully. The element is sulphur (S). Atomic mass number = number of protons + number of neutrons = 16 + 16 = 32 Species is not neutral as the number of protons is not equal to electrons.
Isotopes & Relative Atomic Mass (solutions, examples, videos) Chemistry: How to calculate Atomic Mass of an element, Isotopes, Isotope Notation, Atomic Mass Unit (amu), Relative atomic mass, How to The extra neutrons just change the mass of the atom and its density. Some of the atoms of certain isotopes are unstable because of the extra number of...
Atomic Number Worksheet | PDF | Proton | Neutron Atomic Number Worksheet - Free download as Word Doc (.doc / .docx), PDF File (.pdf), Text File (.txt) or read online for free. atomic number worksheet for group activity. Protons, Neutrons, and Electrons Practice. Worksheet. Atomic Atomic Mass Atomic.
Atomic mass and Atomic number worksheet Chemistry online worksheet for 8th grade. You can do the exercises online or download the worksheet as pdf. All worksheets Only my followed users Only my favourite worksheets Only my own worksheets.
How to Calculate Atomic Mass For a single atom, atomic mass is the sum of the protons and neutrons. Electrons are much smaller than protons and neutrons, so their mass This number usually is given below an element's symbol. Look for the decimal number, which is a weighted average of the atomic masses of all the natural...
Lesson 12: Atoms by numbers atomic number and atomic mass Here is a simple atomic model of a beryllium atom. Label the electrons nucleus, neutrons, and protons. How does the mass of each atom compare to the average atomic mass of the element given in the periodic table? They are very similar in the numbers, but there are little differences.
Isotopes and Atomic Mass - Isotopes | Atomic Mass - PhET... We are working to improve the usability of our website. To support this effort, please update your profile!
Relative Atomic Mass Chemistry Tutorial | mass number (A) Defining relative atomic mass and relative atomic mass calculations tutorial with worked examples for Chemistry students. The mass number (A) of an isotope tells us how many protons and neutrons are in the nucleus of an atom of this isotope. Nucleon is the term used to describe both protons and...
Relative Atomic Mass : GCSE Chemistry The Relative Atomic Mass of an element is the mass of an average atom of that element taking into account its different isotopes and their relative proportions For example, most atoms of the element phosphorus have a mass number of 31 and so may be referred to as phosphorus-31, however, some...
Atomic Structure Worksheets You find three simple sub-atomic particles in each atom. In the center (nucleus) you will find neutrons and protons. Together they provide almost all of the mass This is truly one of the largest collections of atomic structure worksheets in one place. These worksheets have students explore the nature of...
Atomic number, atomic mass, and isotopes (article) | Khan Academy Fundamental properties of atoms including atomic number and atomic mass. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons.
Periodic Table Atomic Mass - Trick To Learn Atomic Mass Number How To Find The Atomic Number Of All Elements In Periodic Table ||Trick To Learn Periodic Table ||.
Atomic number, Mass number and Isotopes - презентация онлайн What are its atomic number and mass number? 1 H 1.00797 • This atomic mass is the one number that best represents the mass of all three versions of hydrogen. • No atom of hydrogen anywhere in the universe actually has this mass. •

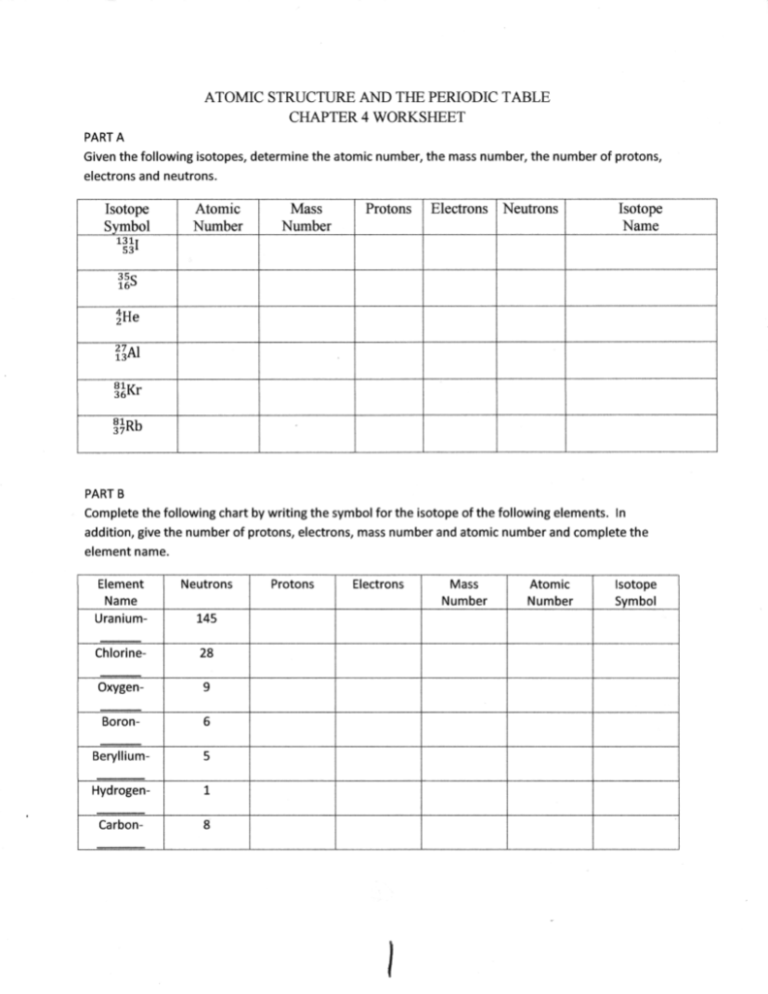

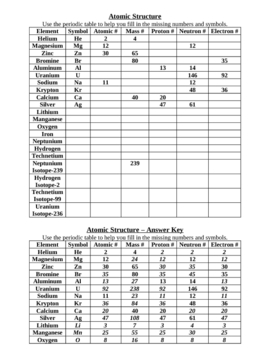

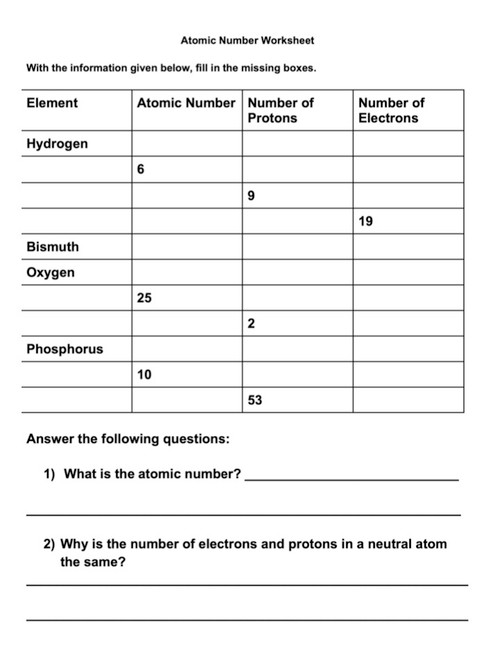

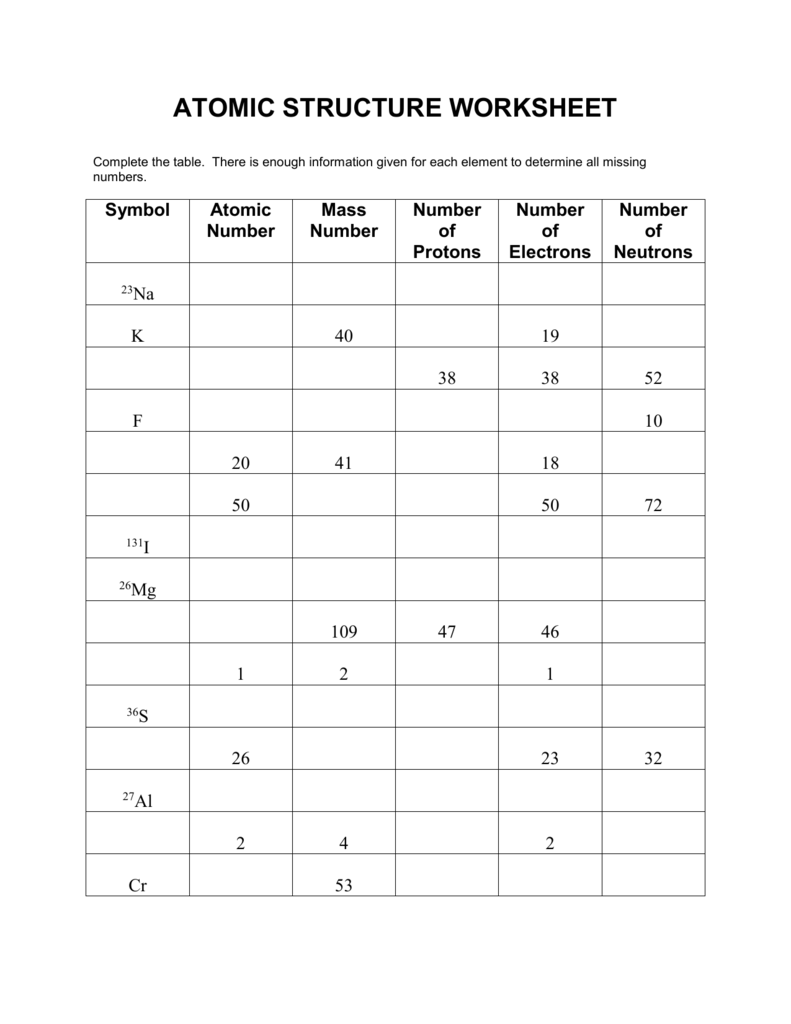















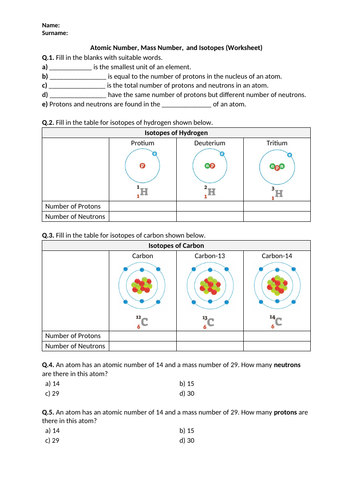

![Atomic Number, Mass Number and Isotopes [Worksheet] by Good ...](https://ecdn.teacherspayteachers.com/thumbitem/Atomic-Number-Mass-Number-and-Isotopes-Worksheet--2519547-1630098518/original-2519547-4.jpg)
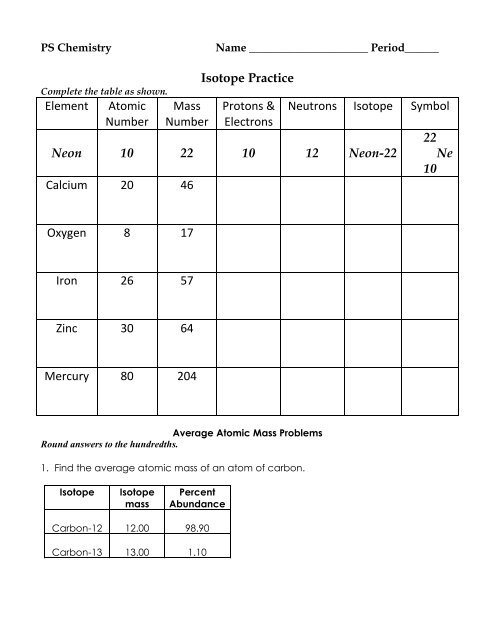
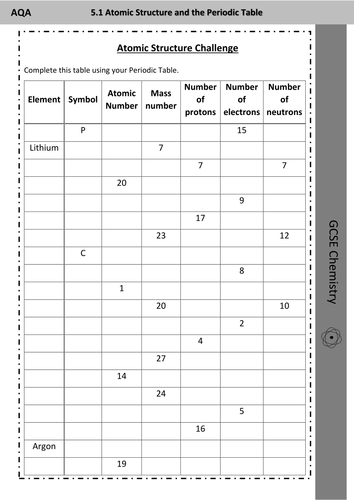
0 Response to "41 Atomic Mass And Atomic Number Worksheet"
Post a Comment