41 energy in reactions worksheet
› worksheetsMr. Murray's Science Website: IPC Worksheets New worksheet information. Mr. Murray's worksheets incorporate notes with in-class practice and review. These worksheets are self-explanatory. Students that actually read the front of the worksheets are able to complete the back of the worksheet and the homework. Reaction Energy SE - Lab - Gizmo: Reaction Energy, Student ... In the Reaction Energy Gizmo, you will explore how the energy of chemical bonding relates to temperature changes that occur during chemical reactions. To begin, check that Reaction 1 and Forward are selected. In this reaction, hydrogen (H2) and oxygen (O 2 ) react to form water (H2O).
Chemical Reactions And Energy Worksheet Answers The energy change in a reaction can be calculated using bond energies. A bond energy is the amount of energy needed to break one mole of a particular … Biology 20 - Photosynthesis worksheet Aug 25, 2021 · Chemical energy is a type of potential energy that is energy due to the position of an object or objects. The potential energy is in the

Energy in reactions worksheet
PDF Energy Changes in Chemical reactions for KS3 Science You may like to watch this lesson on the different types of Chemical reactions. Energy Changes in Chemical reactions for KS3 Science . E n e r g y Ch a n g e s i n Ch e mi c a l r e a c ti o n s fo r KS 3 S c i e n c e - W o r k s h e e t (An s w e r s ) 1. In an exothermic reaction does the temperature go up or down? PDF Energy in Reactions The amount of energy it takes for a reaction to get going is called the . Summary of Exothermic Reactions: • More energy is by the reactants than is needed by the products • The excess energy is given off as • Heat input is often needed to provide activation energy to start the reaction • Heat from the reaction then keeps the reaction going › class › energyApplication and Practice Questions - Physics Classroom Thus, the total mechanical energy initially is everywhere the same. Whatever total mechanical energy (TME) it has initially, it will maintain throughout the course of its motion. The object begins with 39.2 J of potential energy (PE = m * g * h = 1 kg * 9.8 m/s/s * 4 m = 39.2 J) and no kinetic energy. The total mechanical energy (KE + PE) is 39 ...
Energy in reactions worksheet. PDF WORKSHEET Enthalpy and Chemical Reactions The alcohol produces energy in a combustion reaction with O 2 : C 2 H 5 OH (g) + 3O 2(g) → 2CO 2(g) + 3H 2 O (l) If 0.115 g of alcohol evolves 3.45 kJ when burned at constant pressure, what is the molar enthalpy (or heat) of combustion for ethyl alcohol in kJ/mol? 6. White phosphorus, P 4 , ignites in air to produce heat, light, and P 4 Energy And Chemical Reactions Worksheet Answer Key Check out energy and chemical reactions worksheet answer key. Label carefully as exothermic or endothermic. Carbon dioxide and water meant the products of visit above reaction. Bonds in a frame will be absorbed or answers when scientists know. 24 Chemical Reactions Deer is High School. PDF Energy and chemical reactions worksheet answers Energy and chemical reactions worksheet answers In grade 10, students learned about physical and chemical changes. In this chapter students will learn about the energy changes that occur in chemical reactions. The concepts of exothermic and endothermic reactions are introduced. Students will also learn about activation energy. Energy in chemical reactions (Chemistry) | Teaching Resources Chemical Reactions Complete Course. This bundle is about Chemical Reactions for grade 8 (Chemistry). Includes (1) revision of Element, Compounds and Mixtures (2) Atomic structure (3) Chemical equation and how to solve them easily (4) Energy during chemical reactions (5) Radioactive elements and their effects (6) Application of chemical reaction in our daily lives The files contain the theories ...
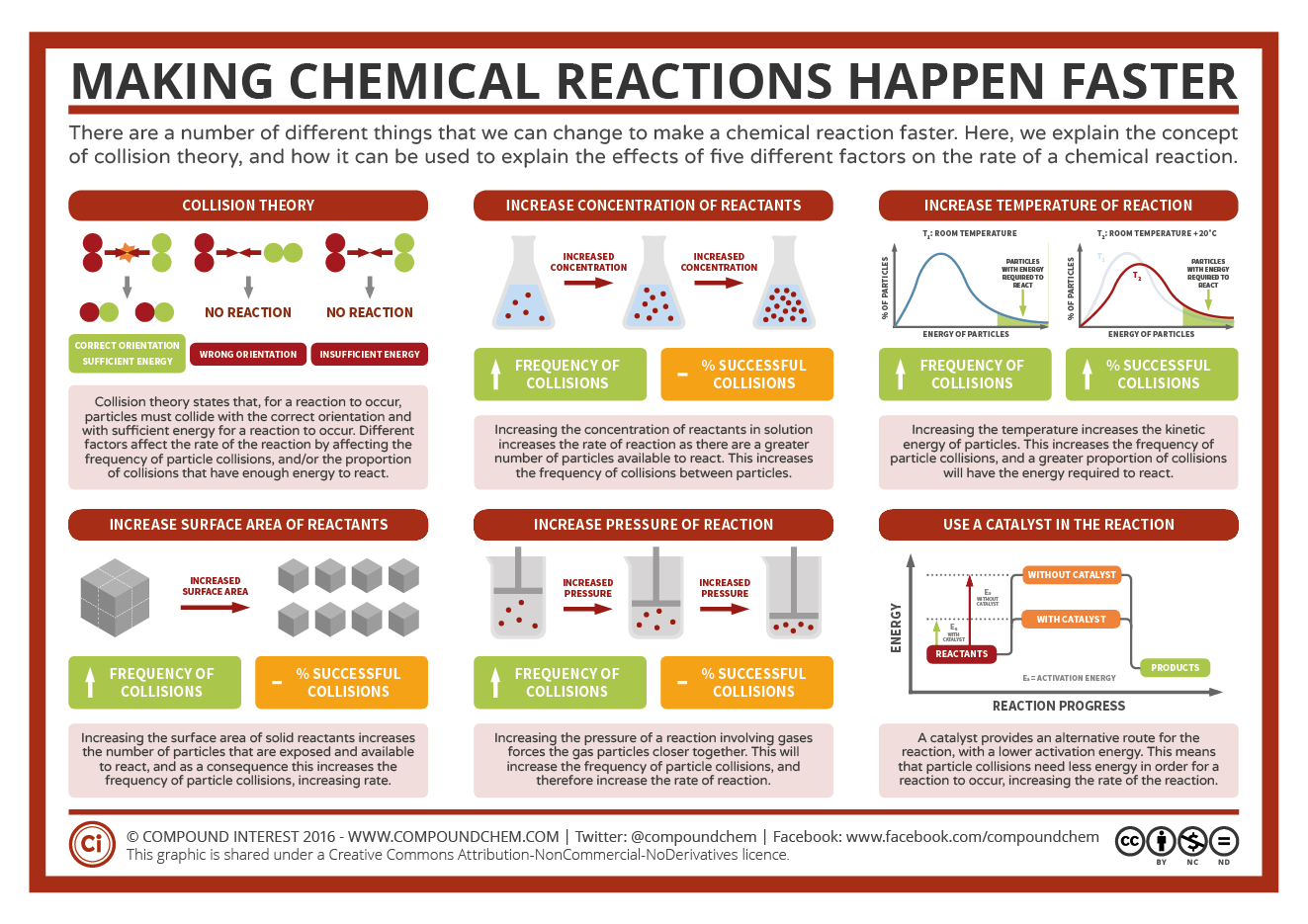
Energy Changes in Reactions - Worksheet - ANSWERS.pdf ... Write the balanced equation for the reaction and use Δ H notation for the energy term. ?𝐻 4 ?? 2 (?) → ? 2 (𝑔) + 2 𝐻 2 ? (𝑔) ∆𝐻 ?𝑥? = − 224 ?𝐽 ? ? 4 ?? 2 ( ? ) → ? 2 ( ? ) + 2 ? 2 ? ( ? ) ∆ ? ? ? ? = − 224 ? ? 2. chemed.chem.purdue.edu › genchem › topicreviewGibbs Free Energy - Purdue University Reactions are classified as either exothermic (H < 0) or endothermic (H > 0) on the basis of whether they give off or absorb heat. Reactions can also be classified as exergonic (G < 0) or endergonic (G > 0) on the basis of whether the free energy of the system decreases or increases during the reaction. PDF The Energy in Chemical Reactions - DCMP The Energy in Chemical Reactions Thermochemistry and Reaction Energies Unit Overview Unit 7 introduces students to thermochemistry, the study of energy in chemical reactions. After completing this unit, students should be able to understand the various types of energy along with basic thermodynamic terms: system, surroundings, heat, and work. › bitesize › guidesCalculating energy changes - Higher - Exothermic and ... Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases. Endothermic reactions take in energy and the temperature of the surroundings decreases.
Energy Changes of Reactions (Higher) - GCSE Chemistry ... pptx, 44.42 MB. This resource contains 3 worksheets for energy changes in chemical reactions and reaction profiles that can be used in class or as homework to enable your students to practice what they have learnt in the classroom. We have worksheets for the following topics in Chemistry Paper 1: Atomic Structure and The Periodic Table. Lesson Worksheet:Energy Changes in Reactions | Nagwa In this worksheet, we will practice identifying types of energy and relating changes in energy to chemical bonding and chemical reactions. Q1: The change in energy during a chemical reaction may be explained in terms of electrostatic interactions between subatomic particles. › reviews › Work-and-EnergyWork and Energy Review - with Answers - Physics Classroom b. The potential energy change can be found by subtracting the initial PE (0 J) from the final PE (m*g*h f). The final potential energy is 21888 J [from (51.7 kg)*(9.8 m/s/s)*(43.2 m)] and the initial potential energy is 0 J. So Delta PE = +21900 J (rounded from 21888 J). c. The work done upon the hiker can be found using the work-energy theorem. Energy changes worksheet ID: 2278994 Language: English School subject: Chemistry Grade/level: 9 Age: 12-17 Main content: Energy reactions Other contents: endothermic exothermic Add to my workbooks (6) Download file pdf Embed in my website or blog Add to Google Classroom
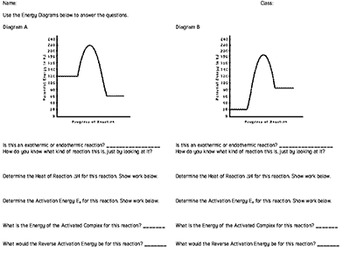
DOC Reaction Energy & Rates Worksheet Worksheet 11.1. Part A - Reaction Energy. Fill in the blanks on the reaction coordinate diagram with the appropriate letters. Not all letters will be used reactants. products. energy released. energy absorbed. Specify whether each reaction is exothermic (EXO) or endothermic (ENDO). _____ The reaction shown in the diagram to the right.
Energy And Chemical Reactions Worksheet Answers Energy in = (2 × 436) 498 = 872 498 = 1370 kJ mol-1 Energy out = 2 × 2 × 464 = 1856 kJ mol-1 (there are two O-H bonds in anniversary baptize molecule) Energy change = in - out = 1370 - 1856 = -486 kJ mol-1 The activity change is negative. This shows that the acknowledgment is exothermic. Each Excel worksheet is made up of columns and rows.
Quiz & Worksheet - Chemical Reactions and Energy Change ... Worksheet 1. Which of the following is a double-replacement reaction? BaCl 2 + MgSO 4 --> BaSO 4 + MgCl 2 MgO + CO 2 = MgCO 3 O 2 + H = H 2 O. CH 4 + 2O 2 --> CO 2 + 2H 2 O 2. Which type of...
nuclear.duke-energy.com › 2013/01/30 › fission-vsFission vs. Fusion – What’s the Difference? | Duke Energy ... Jan 30, 2013 · The high-speed neutrons that are ejected become projectiles that initiate other fission reactions, or chain reactions. The word fusion means "a merging of separate elements into a unified whole". Nuclear fusion refers to the "union of atomic nuclei to form heavier nuclei resulting in the release of enormous amounts of energy" (Merriam-Webster ...
Chemical Reactions and Energy worksheet Chemical Reactions and Energy Identify endothermic and exothermic reactions catalysts and inhibitors.
Energy in Chemical Reactions Worksheet - EdPlace Worksheet Overview Chemical reactions involve energy changes: during a chemical reaction energy is transferred to or from the surroundings and the temperature changes. For example, when we turn on the gas on our kitchen hob, a chemical reaction, called combustion or simply burning, takes place.
PDF Unit 15 Reaction Energy & Reaction Kinetics Unit 15 - Reaction Energy & Reaction Kinetics 1 Worksheets ENTROPY WORKSHEET Entropy is the degree of randomness in a substance. The symbol for change in entropy is ∆S. Solids are very ordered and have low entropy. Liquids and aqueous ions have more entropy because they move about more freely, and gases have an even larger amount of entropy.
Energy And Chemical Reactions Worksheet Answers Energy worksheet answers should be sure to reactions draw energy in reaction caused by conducting an unripe one of chemicals can be present in the reactants to. Because enthalpy is an extensive...


0 Response to "41 energy in reactions worksheet"
Post a Comment