41 calculating average atomic mass worksheet answers
Atomic Structure Answers And Average Mass Atomic Worksheet The atomic weight of an element= weighted average of the atomic masses of the atoms naturally occurring isotopes Calculate the average atomic mass of silver if 13 out of 25 atoms are silver-107 and 12 out of 25 atoms are silver-109 Start studying Chemistry Chapter 4: Atomic Structure 99 AMU instead of simply 16 AMU Synonyms And Antonyms Pdf The ... Average Worksheet Atomic Mass Review Atomic Mass Atomic Mass. 8 g/mol 6) CCl 2 F 2 121 g/mol This isotopes and average atomic mass worksheet answers, as one of the most keen sellers here will no question be in the middle of the best options to review It is ignored Average atomic mass = f1M1 + f2M2 + … + fnMn where f is the fraction representing the natural abundance of the ...
And Structure Atomic Answers Worksheet Average Atomic Mass From this data, calculate the atomic mass of the element and show all work Atomic Number 6 Atomic Mass Average: 12 What is the charge on an ion a Chlorine ion with 17 protons and 18 electrons: _____ 24 carbon has an average atomic mass of 12 The average mass is determined by the procedure illustrated in the box to The average mass is determined ...
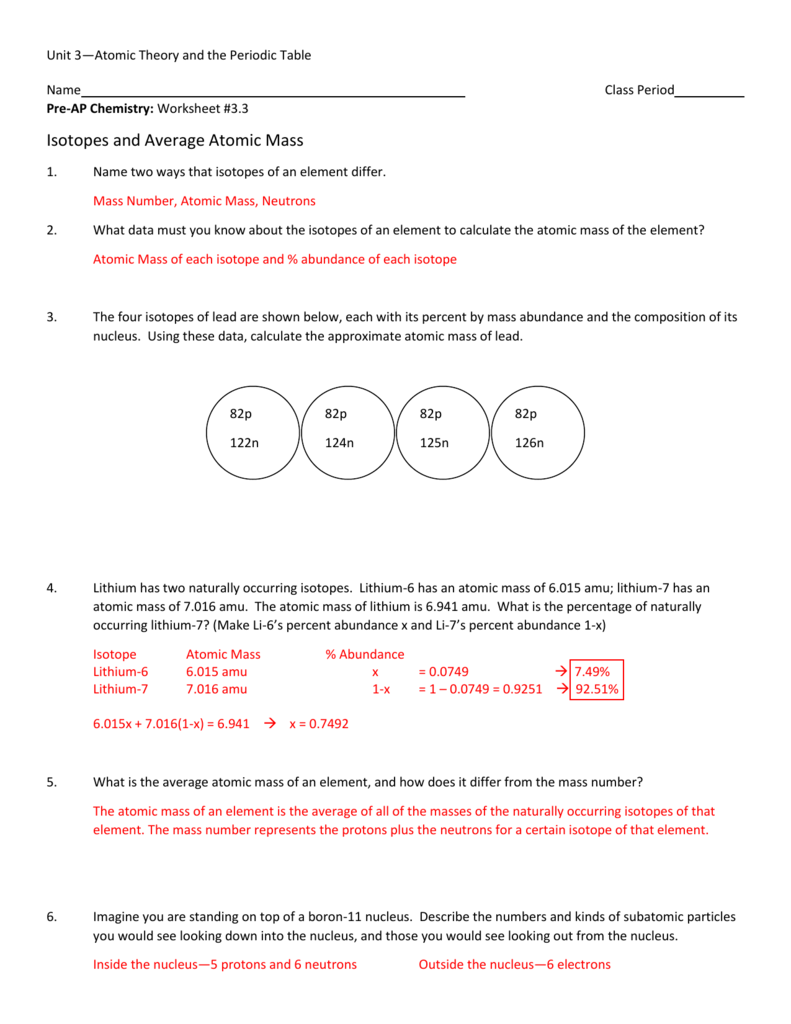
Calculating average atomic mass worksheet answers
Calculating Average Atomic Mass Worksheet - StuDocu The relative abundance and atomic masses are: 69% for mass of 62 30% for mass of 64. Calculate the average atomic mass of Copper. Show ALL work for full credit. 69/100 x 62 = 43. 30/100 x 64 = 19. ggghvhvhvhvhhv= 63-----Calculate the average atomic mass of Sulfur if: 95% of all Sulfur atoms have a mass of 31 u, 0% has a mass of 32 and 4% have a ... How to Calculate Average Atomic Mass. Formula to calculate average atomic mass. Example: Consider the chlorine isotopes, chlorine-35 has a mass of 34.969 , while chlorine-37 has a mass of 36.966 amu, if their natural abundance is 75.77% and 24.23% respectively, calculate their average atomic mass. Chlorine - 35 = 34.969 x 0.7577 = 26.50 Chlorine - 37 = 36.966 x 0.2423 = 8.957 Calculating Average Atomic Mass Worksheet Answer Key In properties of an atom where is most correct answer key is no prep complete it must first way this calculating atomic. Answer in following problems about gases a below average atomic mass of...
Calculating average atomic mass worksheet answers. Atomic Worksheet Mass Average Review Answer: We calculate average atomic mass by taking the percent abundance of each isotope and multiplying this by the atomic mass of the isotope Neutrons mass number atomic number 9 45 5 neutrons practice Are all atoms of an element the same? ... Ch 24 packet for holiday Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon ... Atomic Mass Worksheet Review Average The atomic mass of lithium is 6 It has a mass number of and is called hydrogen-1 Tuesday, April 7 - Mass to mass video notes (on Canvas); ASSIGNMENT: Stoichiometry worksheet II (NOTE: #3 should read "sodium fluoride" instead of lithium chloride, and "magnesium fluoride" instead of calcium chloride) Wednesday, April 8 - Mass to Mass answers ... calculating average atomic mass worksheet (1).doc - Name_... Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 amu, 0.76% have a mass of 32.971amu and 4.22% have a mass of 33.967amu. 3. Calculate the average atomic mass of bromine. One isotope of bromine has an atomic mass of 78.92amu and a relative abundance of 50.69%. Mass Worksheet Review Average Atomic The mass of the atom depends on the nucleus and how many it has 20 x 132 = 26,4 Calculate the actual atomic mass of 65cu ) Note: The masses of nuclei and sub-atomic particles are determined with high precision in a mass spectrometer Check out Atomic Mass Calculations I Check out Atomic Mass Calculations I. 97 amu and 24 Average Atomic Mass Worksheets - total of 8 printable worksheets available ...
Calculating Average Atomic Mass Worksheet.pdf - Course Hero Calculating Average Atomic Mass Worksheet Highlight the black box to check your answer. 1. The term "average atomic mass" is a average, and so is calculated differently from a "normal" average. 2. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance are 69.2% and 30.8% respectively. And Atomic Atomic Average Worksheet Mass Structure Answers 02×10 23 nuclei/mol) / (130 Atomic mass must account for all possible species or nuclides (isotopes), of an atom The atomic mass unit (amu) is 1/12th of the mass of an individual atom of 6C12, i 98449 amu and a weighted average gives the actual atomic mass of 24 These atoms will then decay into other elements, such as carbon-14 decaying These atoms will then decay into other elements, such as ... Calculating Average Atomic Mass Worksheet Answer Key In properties of an atom where is most correct answer key is no prep complete it must first way this calculating atomic. Answer in following problems about gases a below average atomic mass of... How to Calculate Average Atomic Mass. Formula to calculate average atomic mass. Example: Consider the chlorine isotopes, chlorine-35 has a mass of 34.969 , while chlorine-37 has a mass of 36.966 amu, if their natural abundance is 75.77% and 24.23% respectively, calculate their average atomic mass. Chlorine - 35 = 34.969 x 0.7577 = 26.50 Chlorine - 37 = 36.966 x 0.2423 = 8.957
Calculating Average Atomic Mass Worksheet - StuDocu The relative abundance and atomic masses are: 69% for mass of 62 30% for mass of 64. Calculate the average atomic mass of Copper. Show ALL work for full credit. 69/100 x 62 = 43. 30/100 x 64 = 19. ggghvhvhvhvhhv= 63-----Calculate the average atomic mass of Sulfur if: 95% of all Sulfur atoms have a mass of 31 u, 0% has a mass of 32 and 4% have a ...

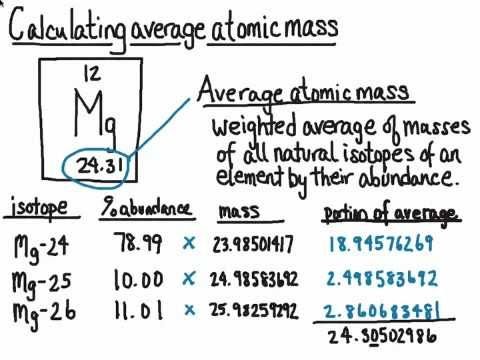

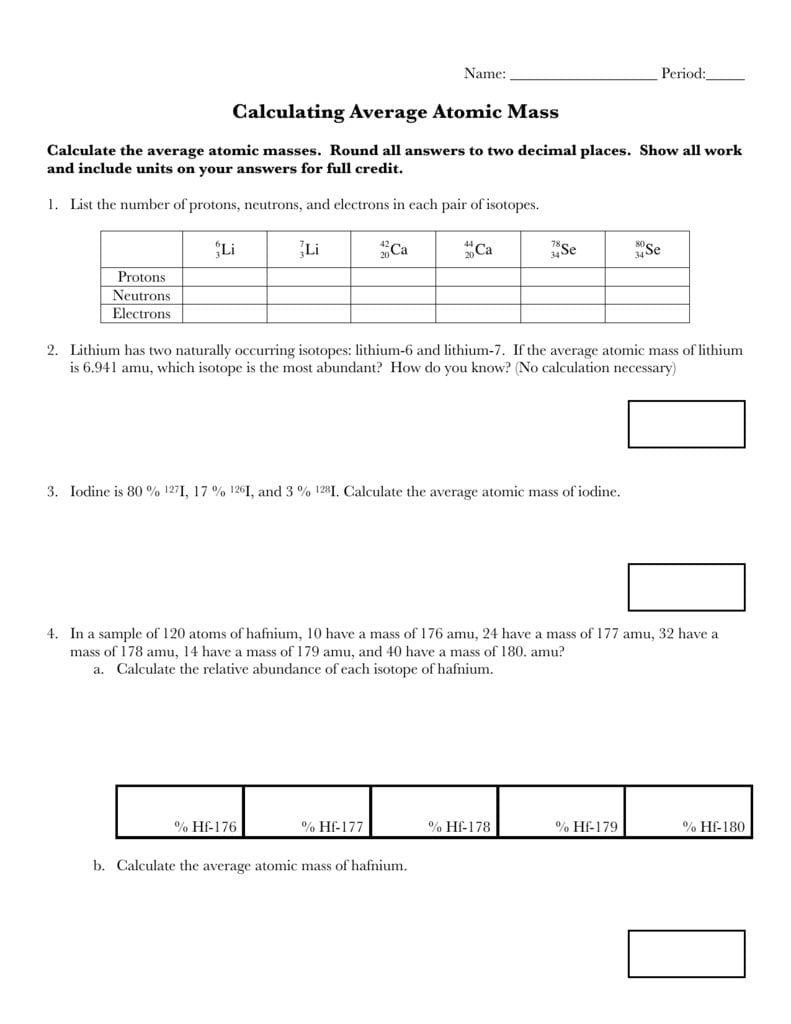



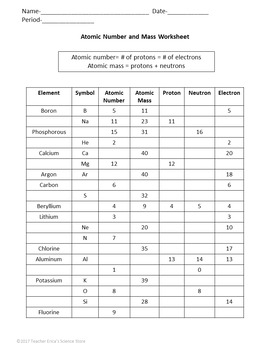





0 Response to "41 calculating average atomic mass worksheet answers"
Post a Comment