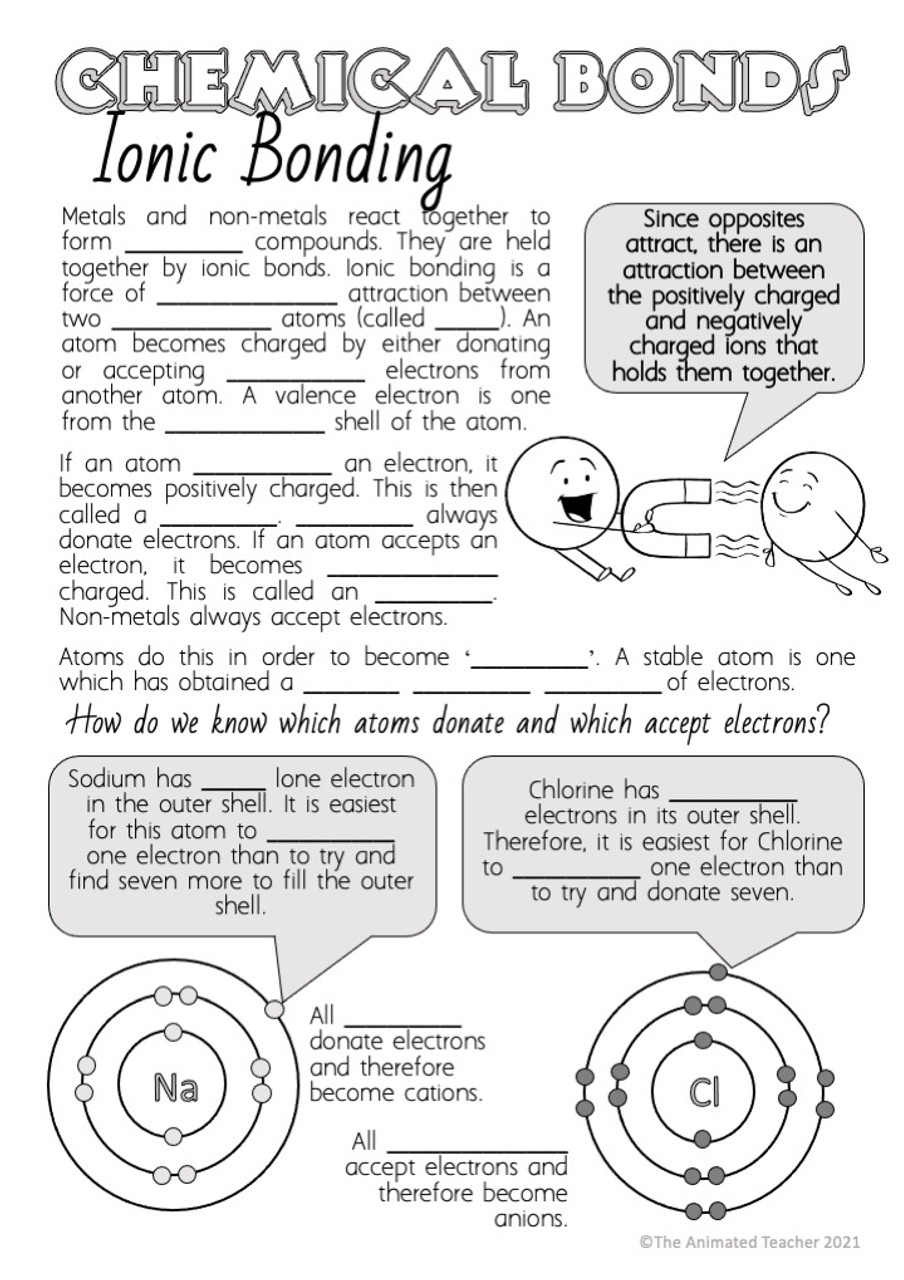
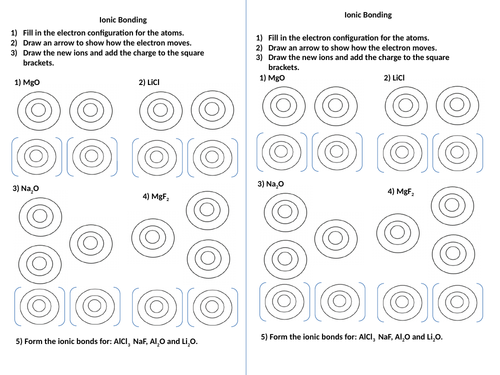
43 atoms and bonding worksheet
The Science Spot Bonding Basics Presentation (ppt) - I use this presentation on my SmartBoard as we complete the Bonding Basics lesson. Bonding Basics Review Worksheet (pdf) - A student worksheet reviewing oxidation numbers as well as ionic and covalent bonding. 2010 Version - Don't have time or materials to make the headbands? Try this one! Carbon Chemistry | Chemistry | Visionlearning The uniqueness of carbon. Carbon (C) appears in the second row of the periodic table and has four bonding electrons in its valence shell (see our Periodic Table module for more information). Similar to other non-metals, carbon needs eight electrons to satisfy its valence shell.Carbon therefore forms four bonds with other atoms (each bond consisting of one of carbon's electrons …
Gas - Wikipedia Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).. A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or compound molecules made from a variety of atoms (e.g. carbon dioxide).A gas mixture, such as air, contains a variety of pure gases.
Atoms and bonding worksheet
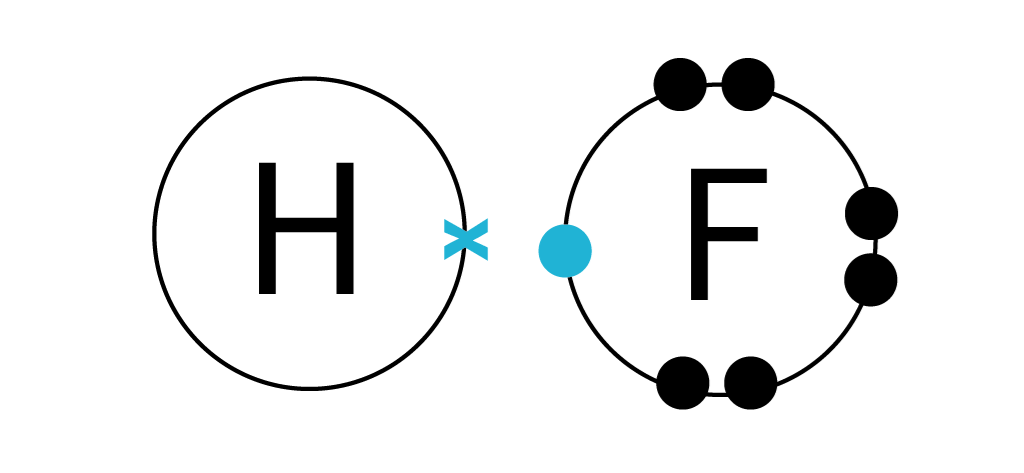
CSUS Chemistry 1A Nomenclature Worksheet Dr. Mack CSUS Chemistry 1A Nomenclature Worksheet Dr. Mack Page 3 of 9 S S2– sulfide ion IA H H– hydride ion Polyatomic Ions Polyatomic ions are ions that are composed of two or more atoms that are linked by covalent bonds, but that still have a net deficiency or surplus of electrons, resulting in an overall charge on the group. Covalent Bonds vs Ionic Bonds - Difference and Comparison | Diffen Covalent bonds are formed as a result of the sharing of one or more pairs of bonding electrons. The electro negativities (electron attracting ability) of the two bonded atoms are either equal or the difference is no greater than 1.7. As long as the electro-negativity difference is no greater than 1.7, the atoms can only share the bonding electrons. Join LiveJournal Password requirements: 6 to 30 characters long; ASCII characters only (characters found on a standard US keyboard); must contain at least 4 different symbols;
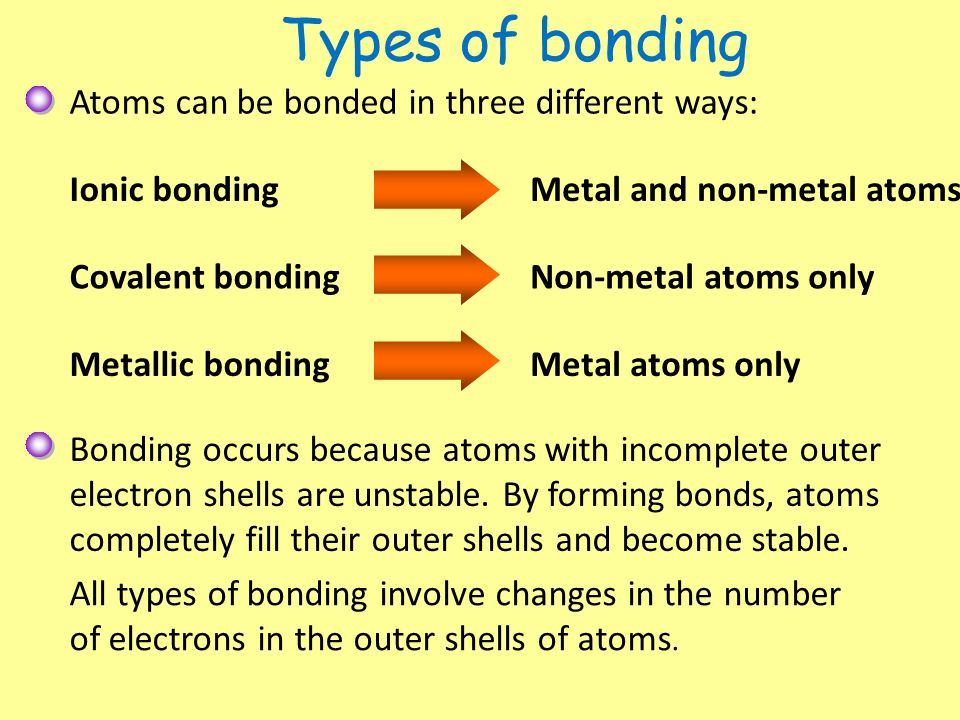
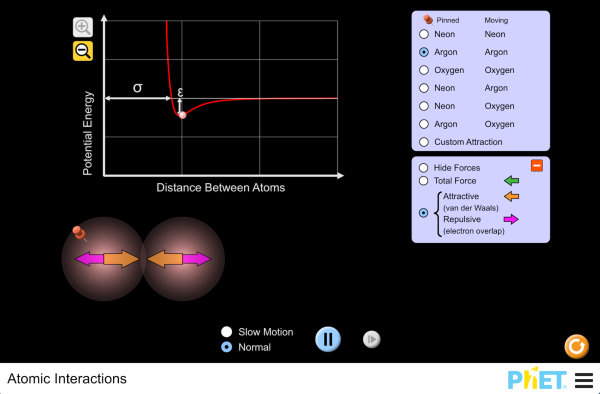
Atoms and bonding worksheet. Valence electron - Wikipedia The number of valence electrons in an atom governs its bonding behavior. Therefore, elements whose atoms can have the same number of valence electrons are grouped together in the periodic table of the elements.. The most reactive kind of metallic element is an alkali metal of group 1 (e.g., sodium or potassium); this is because such an atom has only a single valence … Molecule Shapes - VSEPR | Lone Pairs | Bonds - PhET … Explore molecule shapes by building molecules in 3D! How does molecule shape change with different numbers of bonds and electron pairs? Find out by adding single, double or triple bonds and lone pairs to the central atom. Then, compare the model to real molecules! Thomas Greenbowe | Department of Chemistry and Biochemistry Oct 16, 2017 · 2015–present Senior Instructor II, University of Oregon. 2013–2015 Morrill Professor, Iowa State University. 1998-2013 Professor of Chemistry, Iowa State University. 2013-2014 Visiting Lecturer, University of Oregon. 2006 Visiting Professor, University of Arizona. 1990-1998 Associate Professor, Iowa State University, 1988-1990 Associate Professor of Chemistry and Director of Freshman ... How to Balance Chemical Equations - Study.com Jan 16, 2022 · The equation is not balanced because in the reactants side, there are 2 nitrogen (N) atoms and 2 hydrogen (H) atoms. In the products side, there are 1 nitrogen (N) atoms and 3 hydrogen (H) atoms.
Naming Compounds Practice Worksheet - Loudoun County Public ... D) All exist in nature as individual atoms rather than molecular form. 89) Which of the following statements concerning double covalent bonds is correct? A) They always involve the sharing of 2 electron pairs. B) They are found only in molecules containing polyatomic ions. C) They occur only between atoms containing 4 valence electrons. Join LiveJournal Password requirements: 6 to 30 characters long; ASCII characters only (characters found on a standard US keyboard); must contain at least 4 different symbols; Covalent Bonds vs Ionic Bonds - Difference and Comparison | Diffen Covalent bonds are formed as a result of the sharing of one or more pairs of bonding electrons. The electro negativities (electron attracting ability) of the two bonded atoms are either equal or the difference is no greater than 1.7. As long as the electro-negativity difference is no greater than 1.7, the atoms can only share the bonding electrons. CSUS Chemistry 1A Nomenclature Worksheet Dr. Mack CSUS Chemistry 1A Nomenclature Worksheet Dr. Mack Page 3 of 9 S S2– sulfide ion IA H H– hydride ion Polyatomic Ions Polyatomic ions are ions that are composed of two or more atoms that are linked by covalent bonds, but that still have a net deficiency or surplus of electrons, resulting in an overall charge on the group.
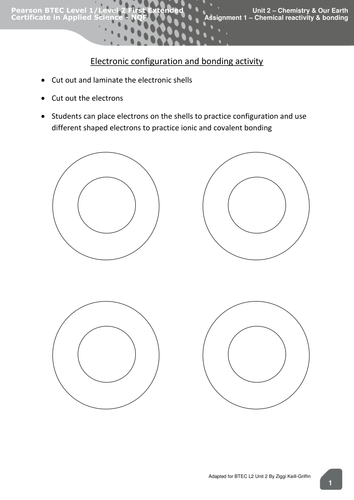




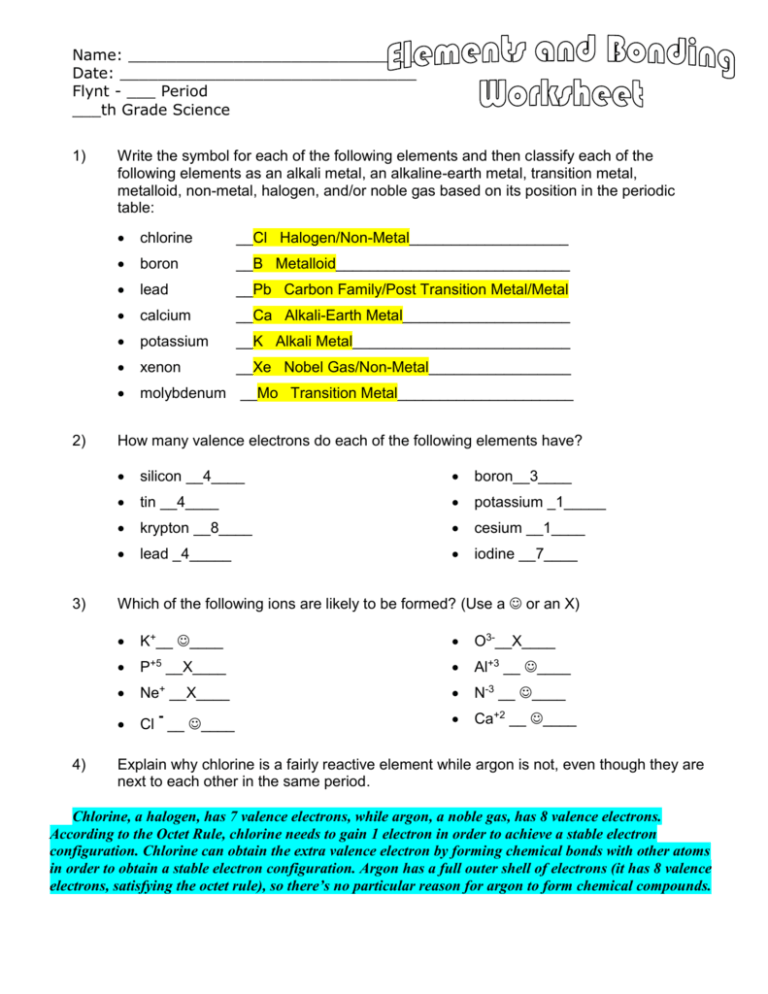

















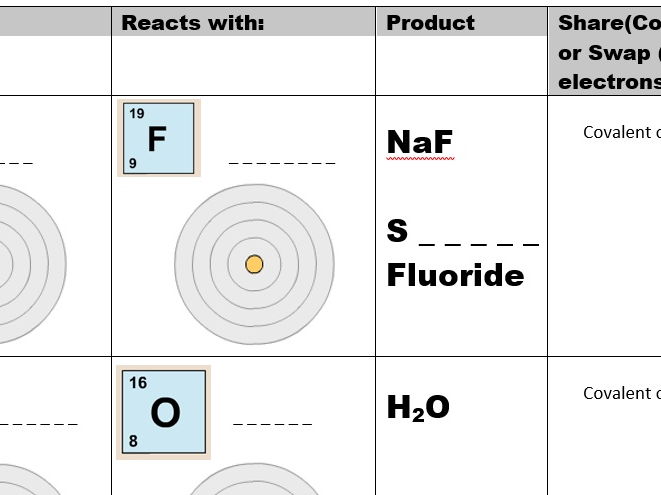






0 Response to "43 atoms and bonding worksheet"
Post a Comment