40 periodic trends atomic radius worksheet
As the elements of Group 1 on the Periodic Table are considered in order of increasing atomic radius, the ionization energy of each successive element generally Decreases The amount of energy required to remove the outermost electron from a gaseous atom in the ground state is known as
G. Practice #2 – Periodic Trends. ATOMIC RADIUS. 1. Does atomic radius increase or decrease as you go down a group/family on the periodic table? increase.
Start studying Chemistry Ch. 11; Periodic Trends Worksheet. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Home. Subjects. Explanations. Create. Study sets, textbooks, questions. ... Compared to the atomic radius of a sodium atom, the atomic radius of a magnesium atom is smaller. The smaller radius is is ...
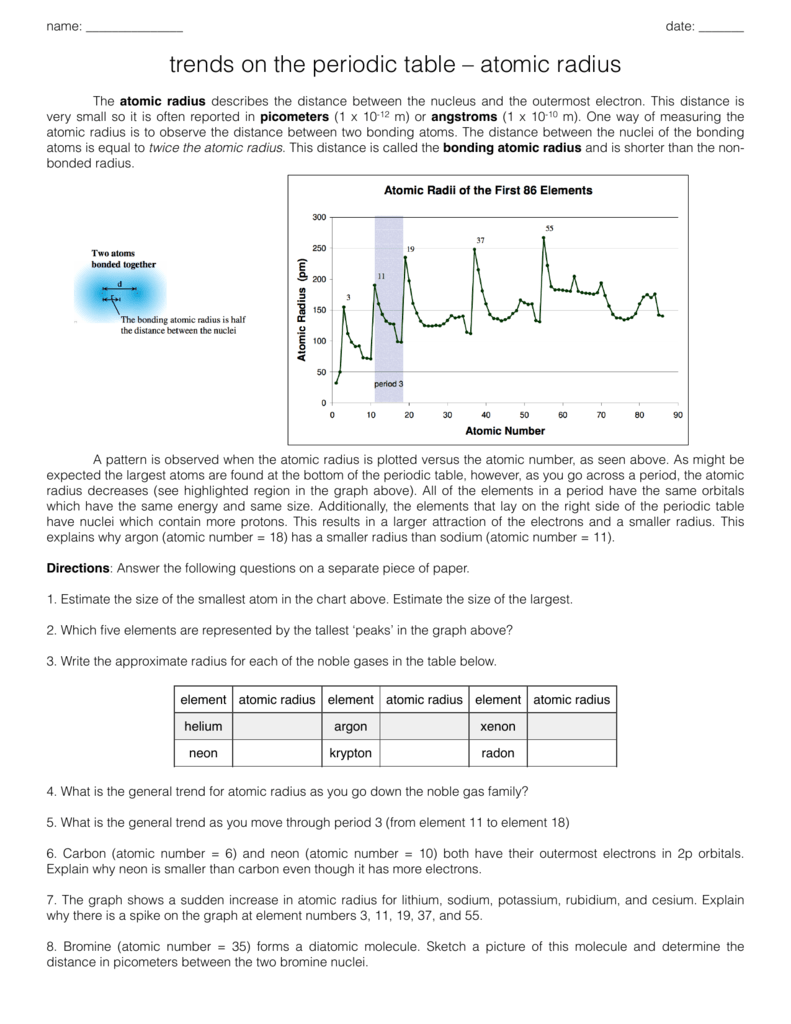
Periodic trends atomic radius worksheet
Periodic Trends Worksheet Define each of the terms and answer the questions that follow. 1. ATOMIC RADIUS: _____ _____ Rank each set of atoms from biggest radius to smallest radius. Set of Atoms Biggest Radius Smallest Radius a. . Li, C,
Periodic Trends. ATOMIC RADIUS. 1. What trend in atomic radius do you see as you go down a group/family on the periodic table? 2. What causes this trend?
This 23 slide chemistry lesson package on the Trends In The Periodic Table discusses Periodic Table Properties, Shielding, Trends, Atomic Radius, Ionic Radius, Ionization Energy and Electronegativity.There is ONE video embedded into the lesson and lots of practice questions to keep your students eng
Periodic trends atomic radius worksheet.
Atomic Size 1) Elements Z and X are compared. Element Z is larger than Element X. Based on this you could say: A) Element Z is further to the left side of the periodic table B) Element X is closer to the top of the periodic table C) Element Z and X are probably in the same group D) A and/or B E) B and/or C 2) Atomic radius generally increases ...
Worksheet from Mon Oct 26 Periodic Trends: Electronegativity, Ionization Energy, Atomic Radius - TUTOR HOTLINE The Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity The Periodic Table: Crash Course Chemistry #4 Periodic Trends Practice Problems: Atomic Radius | Study Chemistry With UsPeriodic Table Trends Trick ...
Created Date: 11/10/2014 3:01:43 PM
Periodic Trends Worksheet Atomic Radius 1. Using the data below, make a bar graph of atomic radius vs. atomic number for Group 2A and for Period 3 of the periodic table. 2 Group 2A Element Atomic Number Atomic Radius Be 4 1.11 Mg 12 1.60 Ca 20 1.97 Sr 38 2.15 Ba 56 2.17 Atomic Radius Atomic Number
Period-Related Pattern Period Number = Number of Energy Levels 3.2 Physical Properties Ionization energy Electronegativity Atomic Radii Ionic Radii Across period 3 & down group 1 & 17 More Trends - Worksheet Ionization Energy: The energy required to remove one valence electron from an atom.
Periodic Trends Worksheet. Directions: Use your notes to answer the following questions. Rank the following elements by increasing atomic radius: carbon, aluminum, oxygen, potassium. Oxygen < Carbon < Aluminum < Potassium. Rank the following elements by increasing electronegativity: sulfur, oxygen, neon, aluminum. Neon < Aluminum < Sulfur < Oxygen
Worksheet 12 - Periodic Trends A number of physical and chemical properties of elements can be predicted from their position in the Periodic Table.Among these properties are Ionization Energy, Electron Affinity and Atomic/ Ionic Radii. These properties all involve the outer shell (valence) electrons as well as the inner shell (shielding) electrons. ...
Worksheet: Periodic Trends 1. ATOMIC RADIUS For each of the following sets of atoms, rank the atoms from smallest to largest atomic radius. a. Li, C, F b. Li, Na, K d. C, N, Al e. Al, Cl, Ga Cli IONIC RADIUS For each of the following sets of ions, rank them from smallest to largest ionic radius. a. Mg , Si b.Mg , ca , Ba c. F, cr, Br d. Ba , cu
Each period in the periodic table corresponds to a(n) a. principal energy level b. energy sublevel c. orbital d. suborbital The modern periodic table is arranged in order of increasing atomic a. mass b. charge c. number d. radius To what category of elements does an element belong if it is a poor conductor of electricity? a. transition elements
Periodic Trends Worksheet Name. The most commonly used measure of size of an atom is its bonding atomic radius also called the covalent radius usually given in units of picometers pm 10 12 m or Ångstroms Å 10 10 m with 1 Å 100 pm. Periodic table trends worksheet. Chemistry periodic trends worksheet answer key Figure PageIndex1.
Periodic trends worksheet answers atomic radius. Groups and periods will be graphed to answer this worksheet. Consider the data in model 1 on the following page. For members of the nitrogen family determine the trend that exists. A li or cs b cl or ar c ca or br d na or ne e b or be 2.
Periodic Trends Worksheet 1 Rank the following elements by increasing atomic radius. Which statement best describes Group 2 elements as they are considered in order from top to bottom of the Periodic Table. Name Period Date Tuesday November 1 2016 Periodic Trends ATOMIC RADIUS 1. Circle the atom in each pair that.
Nov 2, 2020 — Wizer.me free interactive worksheet - Periodic Trends: Atomic Radius Electronegativity by teacher Lynnette Peterson.
26. Draw and label the trends for atomic radius, ionization energy, and electronegativity below Decreasing Increasing Increasing Atomic radius Ionization energy Electronegativity For the test, you should be able to: define or identify the terms related to this unit, explain the periodic trends of
Periodic Trends Worksheet 1) Rank the following elements by increasing atomic radius: carbon, aluminum, oxygen, potassium. 2) Rank the following elements by increasing electronegativity: sulfur, oxygen, neon, aluminum. 3) What is the diffe rence between electron affinity and ionization energy?
Worksheet: Periodic Trends 1. ATOMIC RADIUS For each of the following sets of atoms, rank the atoms from smallest to largest atomic radius. d. C, N, Al e. Al, Cl, Ga 4/ ,/// 64 IONIC RADIUS For each of the following sets of ions, rank them from smallest to largest ionic radius. a. Mg2+, , S2- S S/'
Periodic Trends ATOMIC RADIUS 1. What trend in atomic radius do you see as you go down a group/family on the periodic table? 2. What causes this trend? 3. What trend in atomic radius do you see as you go across a period/row on the periodic table? 4. What causes this trend? 5. Circle the atom in each pair that has the largest atomic radius.
Worksheet 11 - Periodic Trends A number of physical and chemical properties of elements can be predicted from their position in the Periodic Table.Among these properties are Ionization Energy, Electron Affinity and Atomic/ Ionic Radii. These properties all involve the outer shell (valence) electrons as well as the inner shell (shielding) electrons. ...
View HON Periodic Trends Worksheet.pdf from CHM 116 at Missouri State University, Springfield. Periodic Trends Worksheet 1. Rank the following elements by increasing atomic radius: carbon,
Draw in the trends on the periodic table: Ionization energyelectronegativityatomic radiuselectron affinity shielding effect. Atomic Radius. IE / EN* / EA* Periodicity Chemistry Worksheet - page 5. F. Atomic Radius . Circle the atom in each pair with the larger atomic radius? Li or . K. Ca. or Ni Ga or B O or . C. Cl or . Br.
31. $6.99. PDF. This group of activities covers the periodic trends of atomic radius, electronegativity, and ionization energy. In one activity the students measure the atomic radius of several elements going across and down the periodic table. Once the students complete the activity it is easier for them to grasp.
Worksheet: Periodic Trends. 1. ATOMIC RADIUS. For each of the following sets of atoms, rank the atoms from smallest to largest atomic radius. a. Li, C, F.3 pages
Periodic Trends Worksheet #2. Trends-Atomic Radii (size), Ionization Energy, Electronegativity. 1. Circle the one from each pair that would have the larger ...1 page
1. Periodic trends are properties that _____ or _____ going across a period or up or down a group. 2. Below is a rough sketch of the Periodic Table. Sketch in whether the following increase or decrease going across a period, left to right or going up a group: electronegativity, atomic radius, electron affinity, ionization energy.
Periodic Trends Practice Worksheet Answers Written By admin Thursday, July 30, 2020 As one proceeds from left to right across a given period on the periodic table the electronegativities of the elements generally a decrease. Identify each element as a metal metalloid or nonmetal. Describe the trends in the atomic size of elements within groups and across periods in the periodic table.
Periodic Trendsin Atomic Size As you go across a period, the radius gets smaller. Electrons are in same energy level. More nuclear charge. Shielding is constant … not an issue. Outermost electrons are closer. Na Mg Al Si P S Cl Ar GHS Honors Chem Atomic Radius Overall GHS Honors Chem Trends in Ionization Energy
Across a period, the atomic radius decreases because the electrons are added to the ... Periodic Trends Activity. Name: IONIC RADIUS. Ton. Element. Atomic.8 pages
What trend in atomic radius occurs down a group on the periodic table? What causes this trend? What trend in ionization energy occurs across a period on the periodic table? What causes this trend? Circle the atom in each pair that has the largest atomic radius. Al or B. Na or Al. S or O. O or F. Br or Cl. Mg or Ca
Sep 28, 2018 — Why does fluorine have a higher ionization energy than iodine? F smaller atomic radius so tnuclecs can exert a. 4. Why do elements in the same ...4 pages
Rank the following elements by increasing atomic radius. Periodic trends worksheet with answers september 20 2020 september 24 2020 some of the worksheets below are periodic trends worksheet with answers use the periodic table charts and your knowledge of periodic trends to answer several exam style questions like why do atoms get smaller as ...
ANSWER KEY Periodic Trends Worksheet 1. Using the data below, make a bar graph of atomic radius vs. atomic number for Group 2A and for Period 3 of the periodic table.













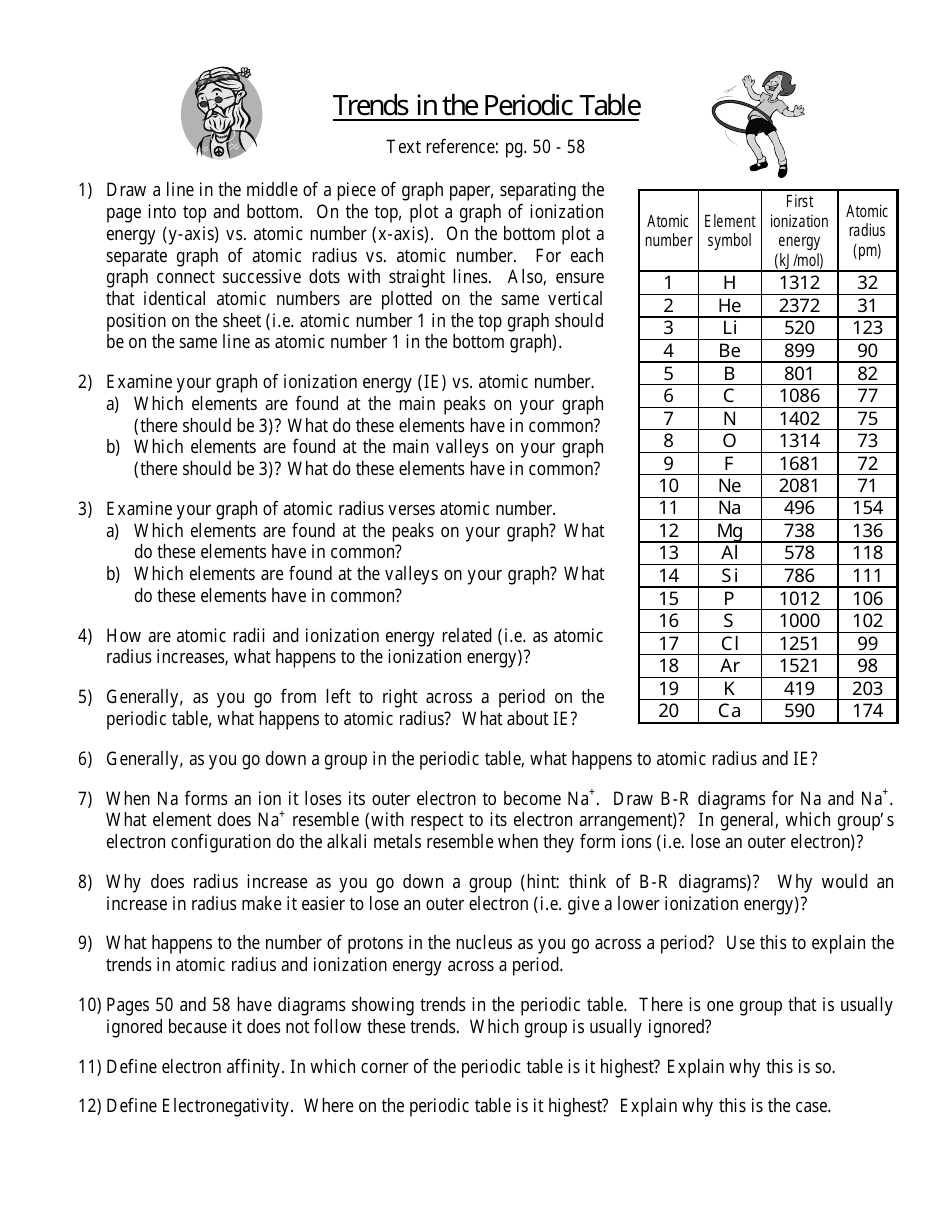









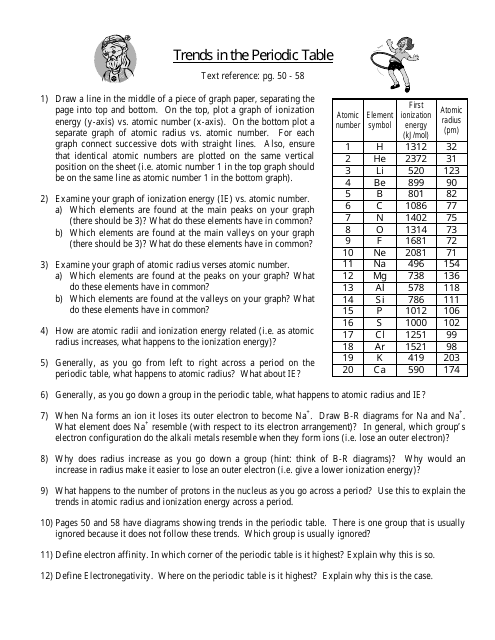









0 Response to "40 periodic trends atomic radius worksheet"
Post a Comment