39 photoelectron spectroscopy worksheet answers
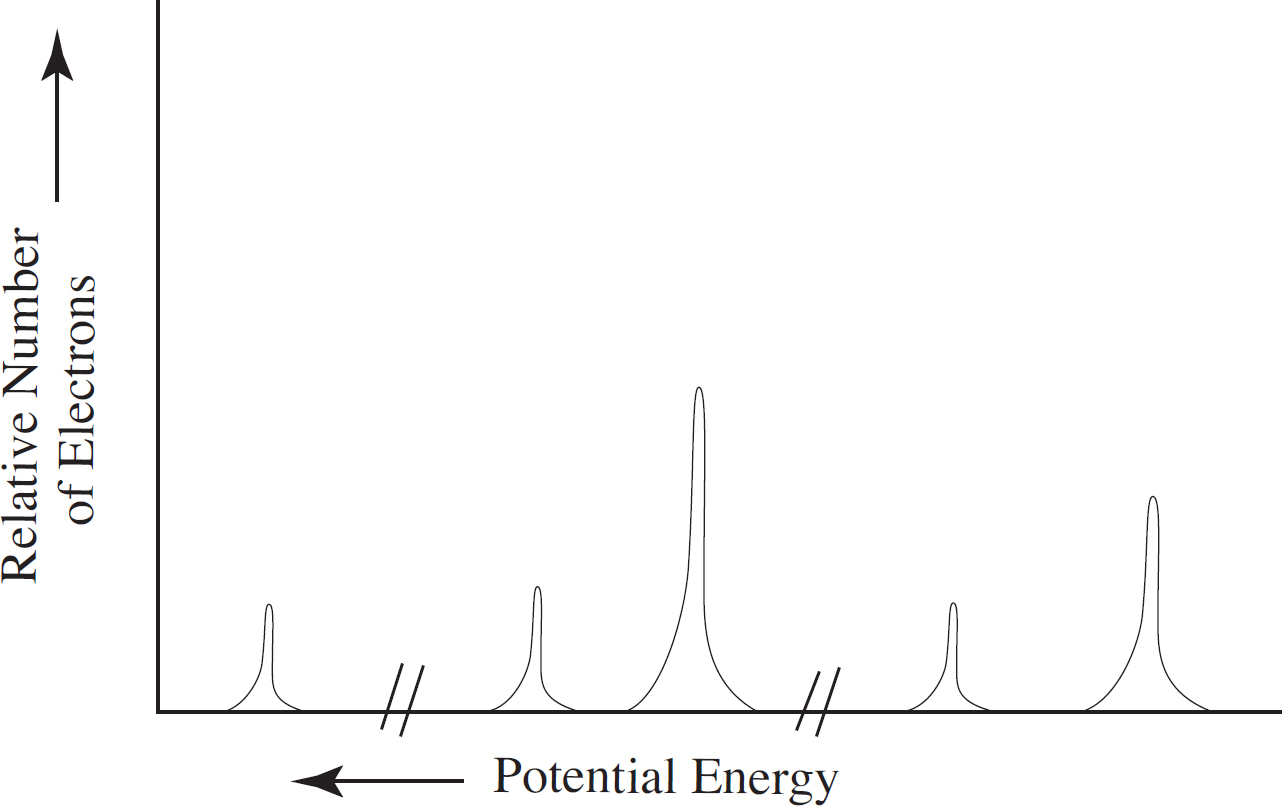
PDF Name: Date: AP Chemistry Big Idea 1 Worksheet: Photoelectron ... - Heroku 1.5 Photoelectron Spectroscopy & Electron Configuration 3. Consider the following PES spectrum a. Using the plot, write the electron configuration of the element, and identify it. b. Label each peak with the appropriate shell and subshell. c. Suggest a reason for the huge jump in energy between peak A and peak B. d. PDF Photoelectron Spectra Samples Photoelectron'Spectroscopy'(PES)Sample'Questions'! ' Questions'113refer'to'the'photoelectron'spectrum'of'neon'shown'below:' ' J. K. Howell R e l a t i v e N u m b e r o f E l e c t r o n s 1000 800 600 400 200 0 Photoelectron Spectrum of Neon Binding Energy (eV) A B C
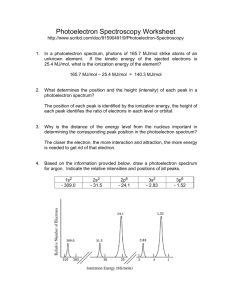
Home / Northern York County Homepage Photoelectron Spectroscopy (PES) ANSWER KEY. In a photoelectron spectrum, photons of 165.7 MJ/mol strike atoms of an unknown element. If the kinetic energy of the ejected electrons is 25.4 MJ/mol, what is the ionization energy of the element? IE = Energy if Photons - KE of electrons = 165.7 MJ/mol - 25.4 MJ/mol = 140.3 MJ/mol

Photoelectron spectroscopy worksheet answers
PDF PES Electron Configuration - Rancho High School 1.5 Photoelectron Spectroscopy & Electron Configuration 5. A student makes the following statement: "Since Ca2+ and Ar, and S2- are isoelectronic, their PES spectra are identical " Is this statement true or false? Justify your answer LvÑt'1 Scorne be becauR pure pnf-nas 6. Write t e e ectron configuration for the followmg elements or ions. PDF Weebly Created Date: 8/27/2015 7:01:34 AM Photoelectron Spectroscopy.docx - Course Hero View Photoelectron Spectroscopy.docx from CHEMISTRY 6.2 at J Sterling Morton West High Sch. Leilani Salgado AP Chemistry 9/27/20 Photoelectron Spectroscopy Questions 1. What determines the position. Study Resources. ... pes_worksheet_with_answers.rtf. Riverside City College. CHEM 2A.
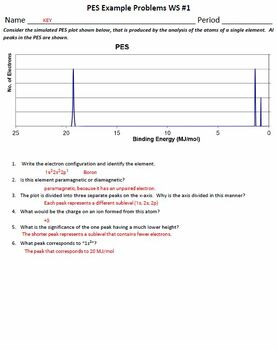
Photoelectron spectroscopy worksheet answers. PDF PHOTOELECTRON SPECTROSCOPY - Pinchas's chemsite 5. Below is shown the PES spectrum of sulfur (atomic number = 16). a. Write the full electron configuration of sulfur. b. Label each peak in the spectrum to show which subshell it represents (i.e., 1s, 2s, etc.) c. On the spectrum, sketch in the relative locations and correct peak heights for the spectrum of aluminum (atomic number = 13). 50 Photoelectron Spectroscopy Worksheet Answers 50 Photoelectron Spectroscopy Worksheet Answers one of Worksheet Preschool Kids - ideas, to explore this 50 Photoelectron Spectroscopy Worksheet Answers idea you can browse by Template and . We hope your happy with this 50 Photoelectron Spectroscopy Worksheet Answers idea. 50 Photoelectron Spectroscopy Worksheet Answers - Chessmuseum 50 Photoelectron Spectroscopy Worksheet Answers. 50 Photoelectron Spectroscopy Worksheet Answers one of Chessmuseum Template Library - free resume template for word education on a resume example ideas, to explore this 50 Photoelectron Spectroscopy Worksheet Answers idea you can browse by Template and . We hope your happy with this 50 ... PDF AP WORKSHEET 01g: Photoelectron Spectroscopy AP WORKSHEET 01g: Photoelectron Spectroscopy 1. Consider the simulated PES plot shown below, that is produced by the analysis of the atoms of a single element. All peaks in the PES are shown. (a) Using the plot, suggest the electron configuration of the element and hence identify the element. (2)
PDF Ap worksheet 01g : photoelectron spectroscopy worksheets answers Photoelectron Spectroscopy 1. (2) (e) The peaks at 1.25 & 2.44, as well as the peaks at 20.2 & 26.8, are relatively close to one another but have different energies? (3) (c) Using your answer in (b), identify the mostly likely charge on an ion of this element. Photoelectron_Spectroscopy_Worksheet-KEY.docx Photoelectron Spectroscopy (PES) ANSWER KEY 1. In a photoelectron spectrum, photons of 165.7 MJ/mol strike atoms of an unknown element. If the kinetic energy of the ejected electrons is 25.4 MJ/mol, what is the ionization energy of the element? IE = Energy if Photons - KE of electrons = 165.7 MJ/mol - 25.4 MJ/mol = 140.3 MJ/mol 2. What determines the position and the height (intensity) of ... PDF 1.6 Photoelectron Spectroscopy AP Chemistry 1.5 Atomic Structure Day 5 ... Photoelectron spectroscopy (PES) allows scientists to determine the ionization energy of not only valence electrons, but all electrons in the atom. In PES, a gaseous sample of atoms is bombarded by X-rays or ultraviolet light (photons) of known energy. The kinetic energies of the photoelectrons that are ejected from the atoms are measured. PDF Photoelectron Spectroscopy Worksheet - Announcements 1. In a photoelectron spectrum, photons of 165.7 MJ/mol strike atoms of an unknown element. If the kinetic energy of the ejected electrons is 25.4 MJ/mol, what is the ionization energy of the element? 165.7 MJ/mol - 25.4 MJ/mol = 140.3 MJ/mol 2. What determines the position and the height (intensity) of each peak in a photoelectron spectrum?
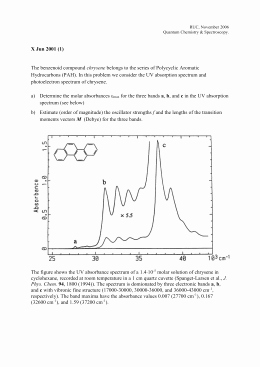
Quiz & Worksheet - Photoelectron Spectroscopy | Study.com Photoelectron spectroscopy is a very useful lab technique and its data can be used in a variety of ways. Test your understanding of photoelectron spectroscopy with this quiz/worksheet combo. The ... Practice Quiz - Photoelectron Spectroscopy - ScienceGeek.net Photoelectron Spectroscopy. Click the "Start Quiz" button to proceed ... PDF 1H & 13C NMR Spectroscopy answers 1H and 13C NMR Spectroscopy Answwers M1.IR Extended response Absorption at 3360 cm-1 shows OH alcohol present Deduction of correct structure without explanation scores maximum of 4 marks as this does not show a clear, coherent line of reasoning. M1 1 NMR There are 4 peaks which indicates 4 different environments of hydrogen PDF Photoelectron Spectroscopy Worksheet - DePauw University Photoelectron Spectroscopy Worksheet Thedatawehaveexaminedthusfarislimitedtoasinglevalenceelectron;thatis,weconsidereddataforan atom'sfirstionizationenergyonly.
Spectra Of Elements Worksheet Answers - Google Groups Answer announce the atoms of an element are excited by absorbing the energy from. Bohr's model does not accurately explain the chemical properties of elements. AP Chem HW 3 Photoelectron Spectroscopy Worksheet 1 In a photoelectron spectrum photons of 1657 MJmol strike atoms of an unknown element If the.
Photoelectron Spectroscopy - AP Chemistry - Varsity Tutors Possible Answers: Correct answer: Explanation: The binding energy at ~ 53 Mj represents the 1s orbital. With other peaks present, this 1s orbital is fully occupied by 2 electrons. The peaks at lower energy would correspond to the 2s and 2p, respectively. Since the 2p peak is twice the intensity of the 2s or 1 s peak, it has twice the electrons (4).
Photoelectron Spectroscopy, Spectrum, PES - AP Chemistry Worksheet Practice This Printable AP Chemistry Worksheet contains carefully selected high-quality multiple-choice questions on Photoelectron Spectrum.A great printable resource to assign to your students for homework, classwork, practice, or review for a quiz, test, or exam.. My resources follow the New AP Chemistry Course Framework.. This worksheet has 34 multiple choice questions in the following two topics of
Photoelectron Spectroscopy Worksheet Answers Photoelectron Spectroscopy Worksheet Answers - Ame Worksheets. Photoelectron Spectroscopy Worksheet Answers. Welcome to our blog, with this time period I'm going to demonstrate about Photoelectron Spectroscopy Worksheet Answers. Think about graphic earlier mentioned? is actually that incredible???. if you're more dedicated therefore, I'l m demonstrate a few picture once again under: So ...
Photoelectron Spectroscopy.docx - Course Hero View Photoelectron Spectroscopy.docx from CHEMISTRY 6.2 at J Sterling Morton West High Sch. Leilani Salgado AP Chemistry 9/27/20 Photoelectron Spectroscopy Questions 1. What determines the position. Study Resources. ... pes_worksheet_with_answers.rtf. Riverside City College. CHEM 2A.
PDF Weebly Created Date: 8/27/2015 7:01:34 AM
PDF PES Electron Configuration - Rancho High School 1.5 Photoelectron Spectroscopy & Electron Configuration 5. A student makes the following statement: "Since Ca2+ and Ar, and S2- are isoelectronic, their PES spectra are identical " Is this statement true or false? Justify your answer LvÑt'1 Scorne be becauR pure pnf-nas 6. Write t e e ectron configuration for the followmg elements or ions.











0 Response to "39 photoelectron spectroscopy worksheet answers"
Post a Comment