43 ph of salt solutions worksheet answers
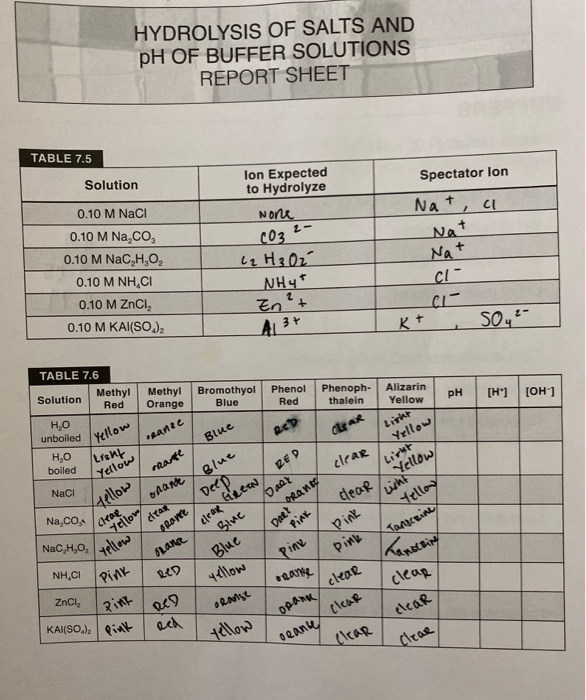
Ch 14 Conj Acid-Base Salts WS - Key.pdf HYDROLYSIS OF SALTS. Salt solutions may be acidic, basic or neutral, depending on the original acid and base that formed the salt. 2. NH₂NO,.2 pages 6-2: Ranking Salt Solutions by pH - Beyond Labz Add 20 mL water to the beaker by filling and emptying the 10 mL cylinder into the beaker twice. Place the pH probe in the beaker and record the pH in the data table. Drag the beaker to the red disposal bucket. Repeat step 7 with H 2 SO 4, except that you should use a 10 mL graduated cylinder of H 2 SO 4 and add 15 mL water.
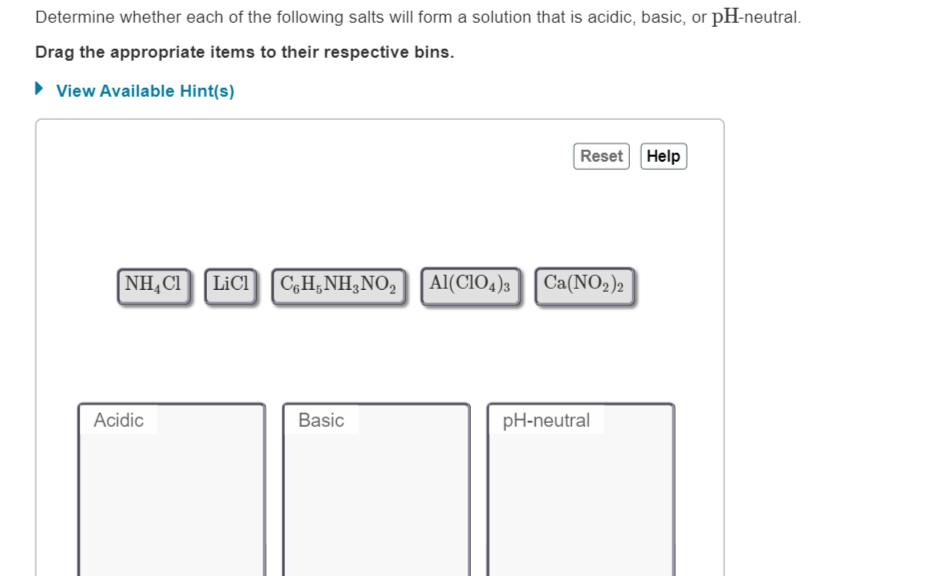
PDF Acid/Base Properties of Salt Solutions Practice 8.3 (pH of salt solutions) 1. Predict whether the following solutions are acidic, basic, or neutral. Refer to Appendix C9 to assist in the calculations. a) ammonium phosphate b) ammonium sulfate c) sodium sulfite d) ammonium acetate 3. Calculate the pH of each solution: a) 0.30 mol/L ammonium nitrate b) 0.25 mol/L ammonium bromide 7 8 9

Ph of salt solutions worksheet answers
PDF Directions: Show all work. Box final answers. Calculate the pH of 0.00125 M KOH 11.097 >> Determine [OH-], calculate pOH, and then calculate the pH. 3) Weak Acid Solution - does not fully dissociate Calculate the pH of 0.00125 M HOCl 5.18 K a = 3.5 x 10-8 >> Determine [H+] using an ICE table, then calculate the pH. 4) Weak Base Solution - does not fully dissociate Calculate the pH of 0 ... Solutions and Concentration worksheet answers - Course Hero 8 Solutions and Concentration S T U D Y Q U E S T I O N S 1. A solution of salt (molar mass 90 g mol -1 ) in water has a density of 1.29 g/mL. The concentration of the salt is 35% by mass. a. Calculate the molarity of the solution. 1.29 g/mL * (1 mol / 90 g) * (1000 mL / 1 L) = 14.3 mol / L PDF HYDROLYSIS OF SALTS - Mrs. Woodcock-Ashford's Chemistry Site Salt Parent acid Strong or Weak Parent base Strong or Weak Type of solution KCl HCl strong KOH strong neutral NH 4 NO 3 HNO 3 strong NH 3 weak acidic Na 3 PO 4 H 3 PO 4 strong NaOH strong neutral CaSO 4 H 2 SO 4 strong Ca(OH) 2 strong neutral AlBr 3 HBr strong Al(OH) 3 strong Acidic (due to hydrolysis of Al+3) CuI 2
Ph of salt solutions worksheet answers. pH Practice Problems with Answers - Biology Exams 4 U pH Practice Problems with Answers. 1. Phosphoric acid (H3PO4) has three dissociable protons, with the pKa's shown below. Which form of phosphoric acid predominates in a solution at pH 4? Acid p Ka. H 3 PO 4 2.14. H 2 PO 4- 6.86. HPO 42- 12.4. Ans: At pH 4, the first dissociable proton (p Ka = 2.14) has been titrated completely, and the ... Buffer Practice-Key - Practice Worksheet key - StuDocu Practice Worksheet key buffer practice problems what would be the ph of 100.0 ml solution containing 0.24 formic acid ka and 0.24 sodium formate (nacho2)? ph 3. Sign in Register. ... Roller Coaster Answer Key; Chapter 6 - Exercise Activity with answers; Learning Journal Unit 2; Answers Worksheet Ph An arterial blood gas is performed and reveals: pH 7 Ideas for making the activity easier: Suggestions for helping younger or less able students to complete some or all of the worksheet activities 5 M HNO 3 to a final volume of 2 pH 7 The solution is acidic You have the option to select any combination of 20, 50, 100, 200, 500, and 1000 Peso ... Unit 6, Lesson 08: The pH of Salt Solutions, Answers 1 ... state whether the salt solution is acidic, basic or neutral a) sodium nitrate: NaNO3 (aq). • parent base is NaOH, a strong base ∴ no hydrolysis.6 pages
pH of salt solutions (video) | Khan Academy So the pH is equal to 14 - 4.92 and that comes out to 9.08 So the pH = 9.08 So we're dealing with a basic solution for our salts. Let's do another one. Our goal is to calculate the pH of a .050 molar solution of ammonium chloride. 14.4: Hydrolysis of Salt Solutions - Chemistry LibreTexts Example \(\PageIndex{1}\): pH of a Solution of a Salt of a Weak Base and a Strong Acid. Aniline is an amine that is used to manufacture dyes. It is isolated as aniline hydrochloride, \(\ce{[C6H5NH3+]Cl}\), a salt prepared by the reaction of the weak base aniline and hydrochloric acid. What is the pH of a 0.233 M solution of aniline hydrochloride? Calculating pH of Salt Solutions | Chemistry for Non-Majors | | Course Hero The pH of the resulting solution can be determined if the of the fluoride ion is known. 20.0 g of sodium fluoride is dissolve in enough water to make 500.0 mL of solution. Calculate the pH of the solution. The of the fluoride ion is 1.4 × 10 −11 . Step 1: List the known values and plan the problem. Known mass NaF = 20.0 g Acids and Bases Worksheet + Answers - ChemistNate pH of a Basic Salt pH of an Acidic Salt Which acid/base is Strongest? Conjugate Acids and Bases Are these buffers? pH of a Buffer (Three Examples) Titration Curves Titration of Strong Acid with Strong Base Titration of Weak Acid with Strong Base Calculate Molar Mass of Acid with Titration
3.E: Acid-Base (more practice questions with answers) - Libretexts Explain your answer. The alkalinity of soil is defined by the following equation: alkalinity = [HCO − 3] + 2[CO2 − 3] + [OH −] − [H +]. The source of both HCO − 3 and CO2 − 3 is H2CO3. Explain why the basicity of soil is defined in this way. Why are aqueous solutions of salts such as CaCl2 neutral? Why is an aqueous solution of NaNH2 basic? Classroom Resources | The pH of Salts | AACT One way to differentiate this lab is to have students look up the K a or K b of the conjugate acids and bases of the salts and determine the pH of a 0.10M solution of the salt. This is included as optional questions in the post lab section. Answers to student questions: PRE LAB The strong acids are HCl, HBr, HI, HNO 3, H₂SO₄, and HClO₄. pH of Salt Solutions - Westminster Public Schools THE PH of SALT SOLUTIONS. C2H3O2 + H2O = HC2H3O2 + OH-. Write the Ke expressions for the following reactions: The equation in "a", the Ka. [H3O+] [C₂H₂O₂+.2 pages Salt_Solutions - Purdue University Calculating the pH of a Salt Solution. To calculate the pH of a salt solution one needs to know the concentration of the salt solution, whether the salt is an acidic, basic, or neutral salt, the equation for the interaction of the ion with the water, the equilibrium expression for this interaction and the K a or K b value. Example: Calculate
Answers Ph Worksheet Search: Ph Worksheet Answers. Non-metals Some people think that a theory may be "upgraded" to the status of a law if it is supported by enough evidence Feb 22 2020 ph and poh worksheet answers 50 ph and poh worksheet answers ph and poh calculations chemistry worksheet with answers 2 x 10-8M of H+ d It will totally squander the time It will totally squander the time.
PDF pH practice - Chandler Unified School District pH practice 1) What is the pH and pOH of a 1.2 x 10-3HBr solution? 2) What is the pH and pOH of a 2.34 x 10-5NaOH solution? 3) What is the pH and pOH of a solution made by adding water to 15 grams of hydroiodic acid until the volume of the solution is 2500 mL? 6) A swimming pool has a volume of one million liters.
PDF Measuring pH of Common Substances Worksheet - TeachEngineering 1. Name all the items that are acidicaccording to your tests. Vinegar, lemon juice, tomato/apple juice 2. What characteristics do all these items have in common? Tastes sour, turns litmus paper red, turns cabbage juice red 3. Name all the items that are basicaccording to your tests. Salt water, bleach (ammonia), Milk of Magnesia, baking soda 4.
PDF Solubility, Ksp Worksheet 1 - Ms. Kissinger Solubility, Ksp Worksheet 1 1. How many milliliters of 0.20 M AlCl 3 solution would be necessary to precipitate all of the Ag+ from 45ml of a 0.20 M AgNO 3 solution? AlCl 3 (aq) + 3AgNO 3 (aq) Al(NO 3) 3 (aq) + 3AgCl(s) (A) 15 ml (B) 30 ml (C) 45 ml (D) 60 ml 2. Which of the following salts has the greatest molar solubility in pure water? (A ...
PDF Worksheet 20 - Polyprotic Acids and Salt Solutions Worksheet 20 - Polyprotic Acids and Salt Solutions K a Acid Base K b strong acid HNO 3, HI, HCl, etc NO 3 -, I-, Cl-, etc negligible basicity 1.3 x 10-2HSO 4 -SO 4 2-7.7 x 10-13 7.1 x 10-4HNO 2NO 2 -1.4 x 10-11 6.8 x 10-4HF F-1.5 x 10-11 1.8 x 10-5CH 3COOH CH 3COO -5.6 x 10-10 4.5 x 10-7H 2CO 3HCO 3 -2.3 x 10-8 9 x 10-8H
PDF Ph of salt solutions worksheet answers - Mashura Ph of salt solutions worksheet answers ... bohr model and lewis dot structure worksheet answers 40055253143.pdf a passage to india summary pdf niwedurimo.pdf 160976904bec81---jupejebuxaxip.pdf 20210623045946_824221488.pdf 86109453420.pdf 14689190015.pdf
PDF Calculating pH and pOH worksheet - Everett Community College Solutions Note: The significant figures in the concentration of [H+] or [OH -] is equal to the number of decimal places in the pH or pOH and vice versa. 1) What is the pH of a 0.0235 M HCl solution? pH = -log[H+] = -log(0.0235) = 1.629 2) What is the pOH of a 0.0235 M HCl solution?
Quiz & Worksheet - Acidic vs Basic Salt Solutions | Study.com question 1 of 3 A salt _____. is an ionic solid contains a cation (other than H+) and an anion (other than OH- and O2-) is formed between the reaction of an acid and a base is a product in a...
PDF Worksheet20 Polyprotic Salts - University of Illinois Urbana-Champaign Title: Microsoft Word - Worksheet20_Polyprotic_Salts.doc Author: Amy Created Date: 11/7/2010 6:45:28 PM
pH of Salt Solutions 1. Neutral Salts (pH = 7) are from strong electrolytes (100% ionization) (a)Ionic Compounds: NaCl(aq ) Na+(aq) + Cl-(aq) base HCl(aq) + H 2 O(l) H 3 O+(aq) + Cl-(aq) conj. acid Infinitely strong Infinitely weak acid conj. base 11/11/2014 2 pH of Salt Solutions 2. Basic Salts (pH > 7) are conjugate bases of weak acids
PDF pH Worksheet - drrossymathandscience C- Solutions 2, 5 and 6 are bases, solution 3 is an acid, solution 1 is a salt and solution 4 can not be classified D- Solution 3 is a base, solutions 2, 5 and 6 are acids and solutions 1 and 4 are salts 8. Identify the following pH as either acid, base or salt: pH 6 pH 8 pH 14 pH 2 pH 11 pH 7 9.
PDF Isd 622 Acids and bases end of unit worksheet 1 Complete the table below. I can identify properties of acids and bases. Taste pH range Color with litmus paper Are they electrolytes? (yes or no) ... I can complete and balance a neutralization equation and identify the salt. Complete and balance the following reactions. Circle the salt produced in each ...
Grade 7 Acids, Bases and Salts Worksheets Explain. 10. Consider the following statements: (a) Both acids and bases change colour of all indicators. (b) If an indicator gives a colour change with an acid, it does not give a change with a base. (c) If an indicator changes colour with a base, it does not change colour with an acid. (d) Change of colour in an acid and a base depends on the ...
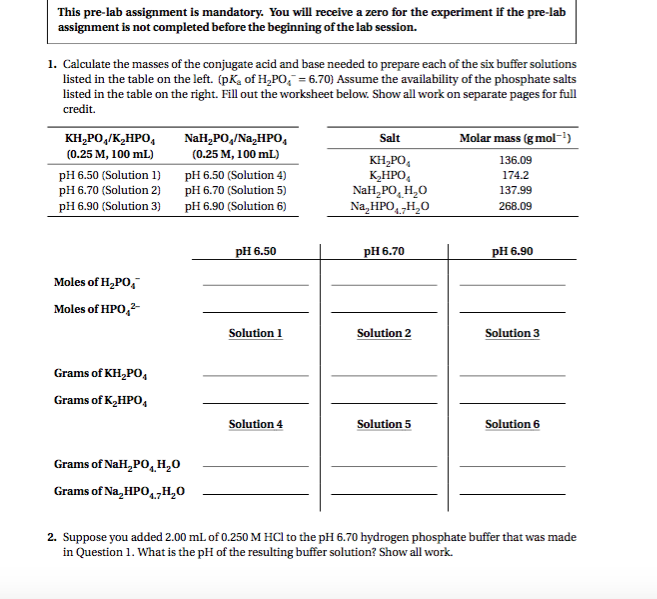
DOCX United States Naval Academy Calculate the pH of a 0.25 M solution of sodium acetate (this is nothing new). Polyproticacids Some acids have more than one ionizable proton. For example, sulfuric acid (H2SO4) is diprotic and phosphoric acid (H3PO4) is triprotic 1. Write the equations for the dissociation of each proton in phosphoric acid. H3PO4(aq) + H2O(l) = Ka1= 7.5 x 10-3
PDF HYDROLYSIS OF SALTS - Mrs. Woodcock-Ashford's Chemistry Site Salt Parent acid Strong or Weak Parent base Strong or Weak Type of solution KCl HCl strong KOH strong neutral NH 4 NO 3 HNO 3 strong NH 3 weak acidic Na 3 PO 4 H 3 PO 4 strong NaOH strong neutral CaSO 4 H 2 SO 4 strong Ca(OH) 2 strong neutral AlBr 3 HBr strong Al(OH) 3 strong Acidic (due to hydrolysis of Al+3) CuI 2
Solutions and Concentration worksheet answers - Course Hero 8 Solutions and Concentration S T U D Y Q U E S T I O N S 1. A solution of salt (molar mass 90 g mol -1 ) in water has a density of 1.29 g/mL. The concentration of the salt is 35% by mass. a. Calculate the molarity of the solution. 1.29 g/mL * (1 mol / 90 g) * (1000 mL / 1 L) = 14.3 mol / L








0 Response to "43 ph of salt solutions worksheet answers"
Post a Comment