44 atomic mass and atomic number worksheet answers
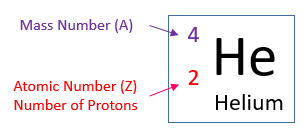
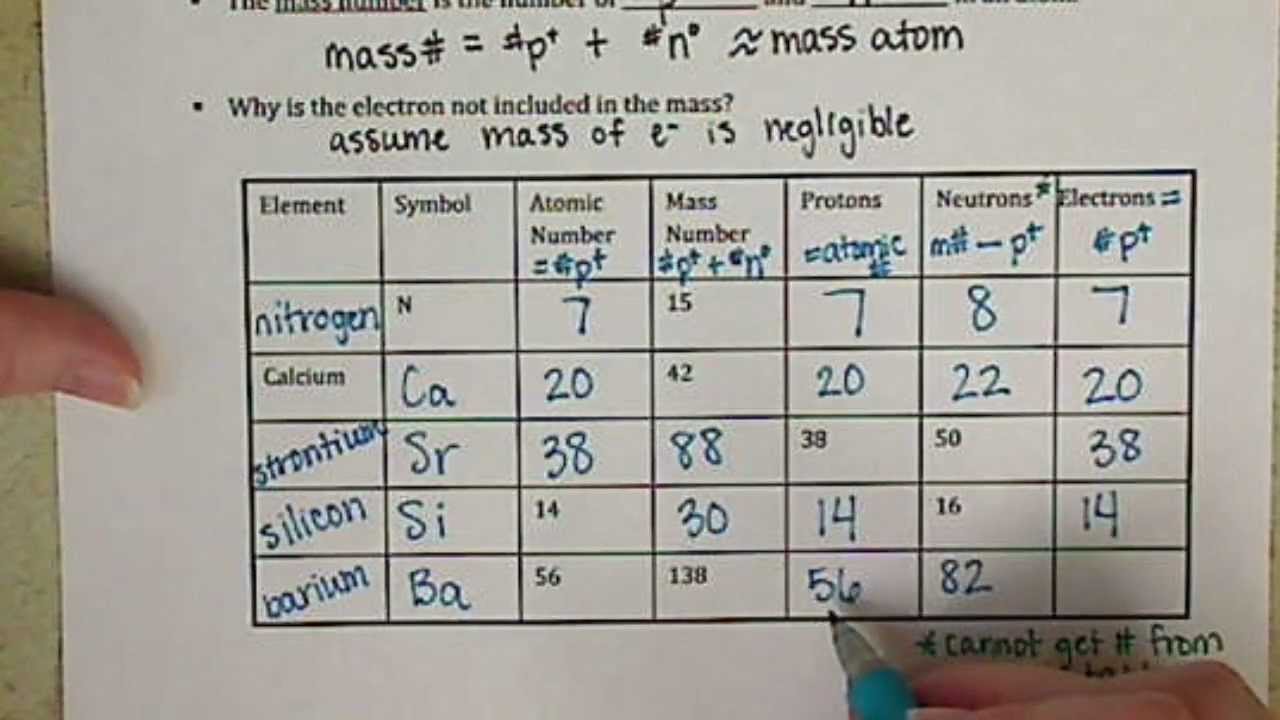
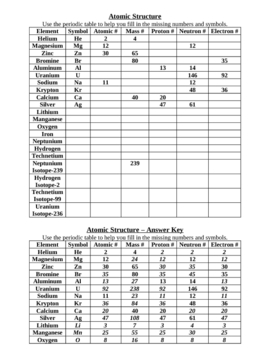
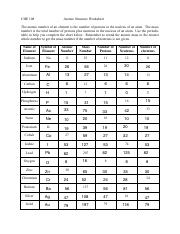
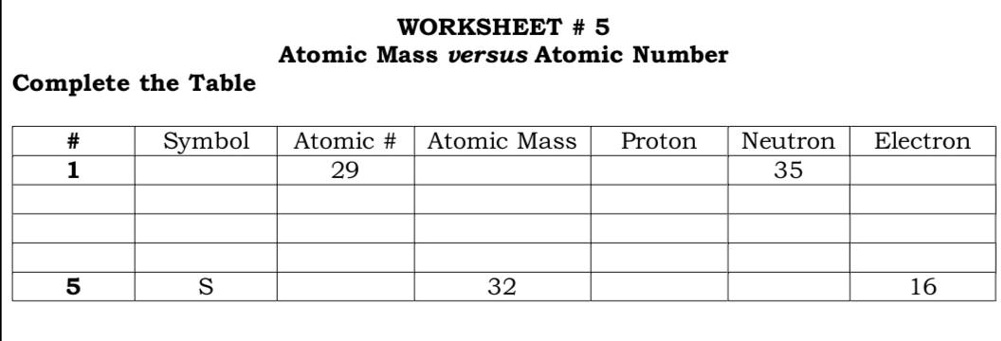
The Atoms Family Atomic Math Challenge Atomic Mass. # of Protons = = # of Neutrons. = # of Electrons = 30. 5. 65. 30. 35. 30. //. 5. 6. PROTONS. ANSWER KEY. Atomic number equals the number of. 9PS - Atomic Number, Mass Number, Isotopes, and Stuff Atomic Number, Mass Number, Isotopes, and Stuff. 1 Complete the following questions. Assume all atoms are neutral. 56. 26. Fe. 2. He element: # protons:.
Atomic Structure Worksheet Answers - Columbia Public Schools Atomic Structure Worksheet Answers - Columbia Public Schools
Atomic mass and atomic number worksheet answers
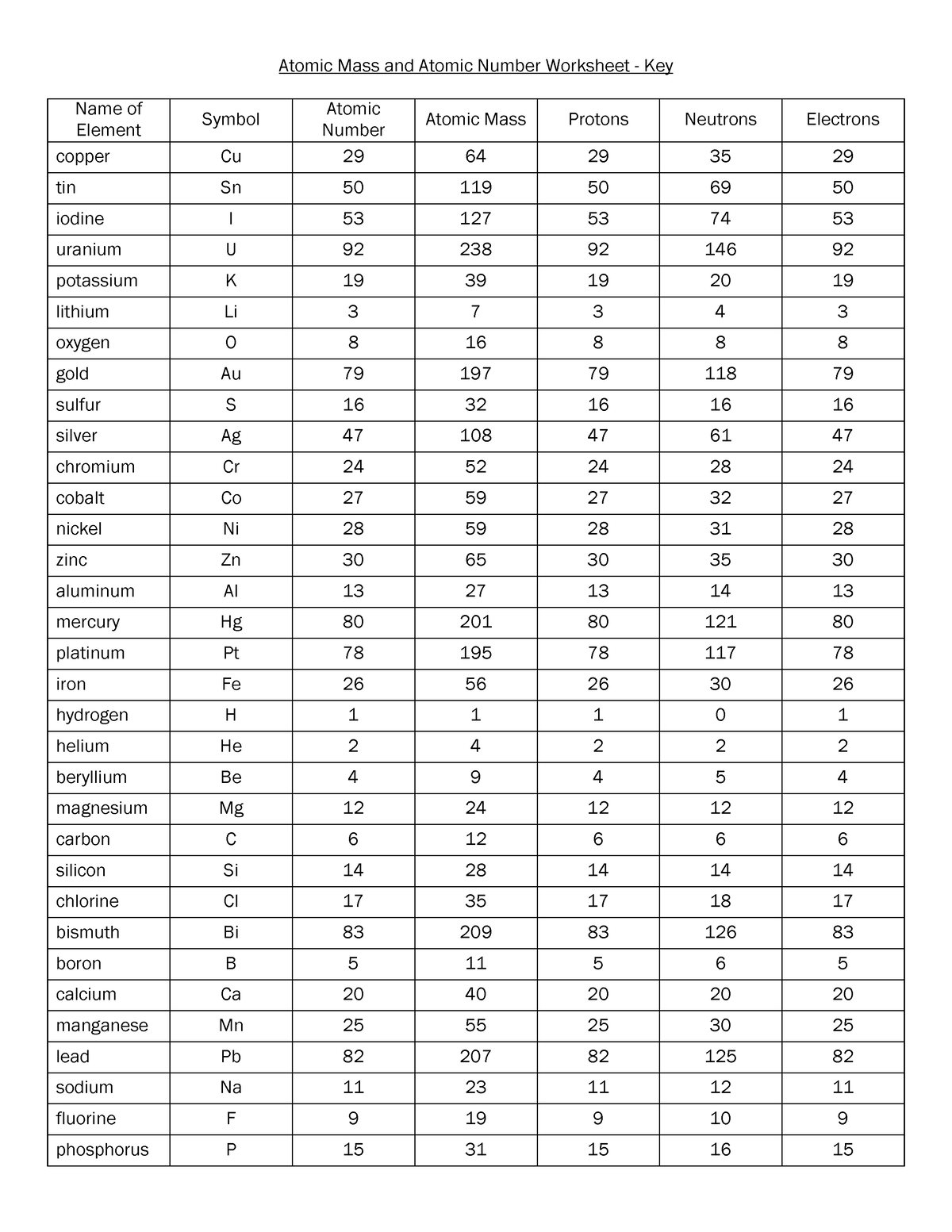
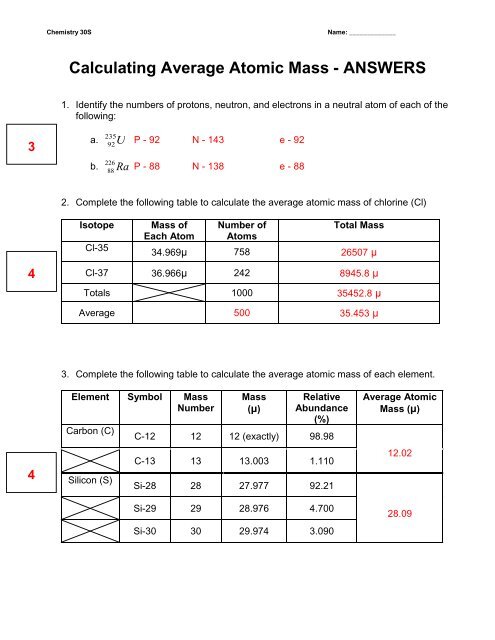
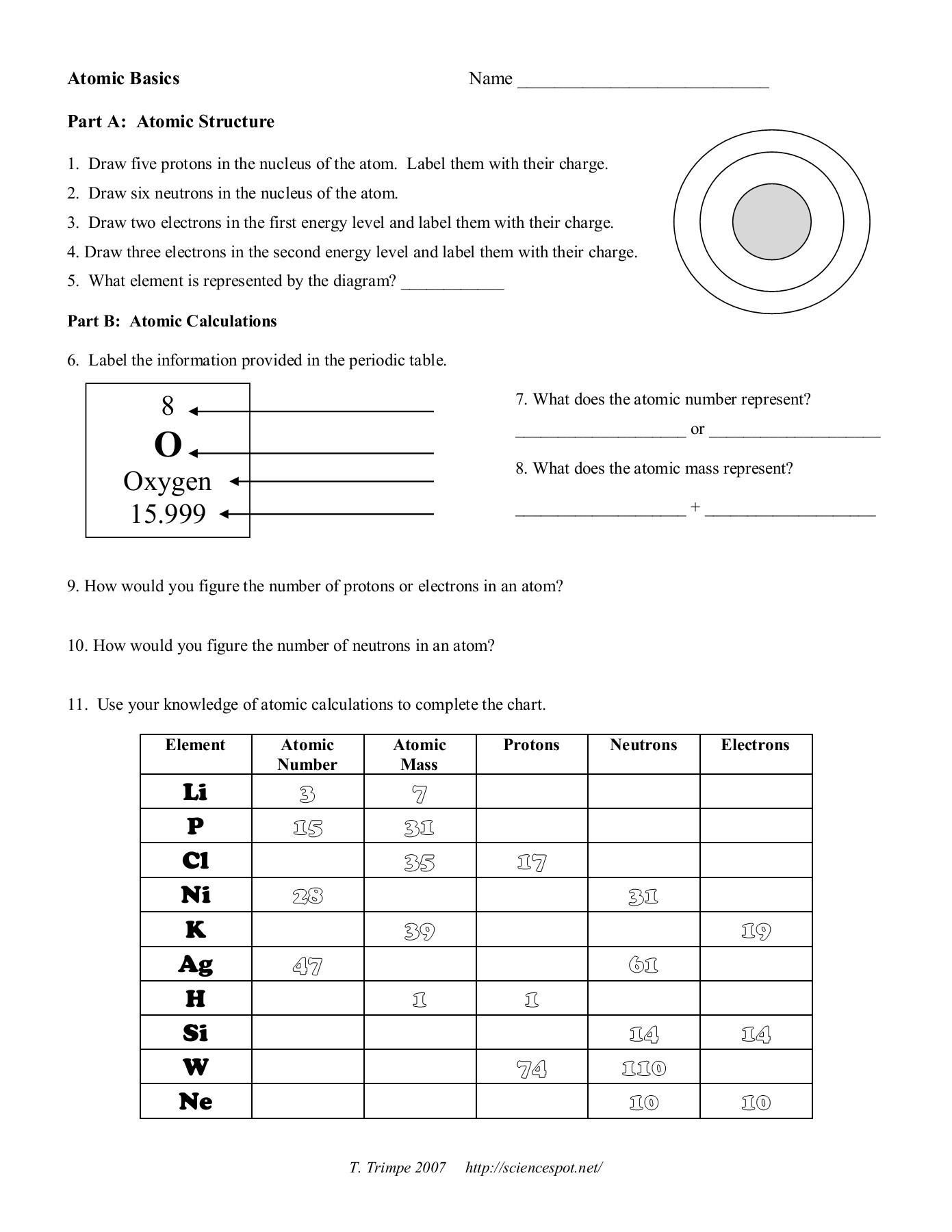
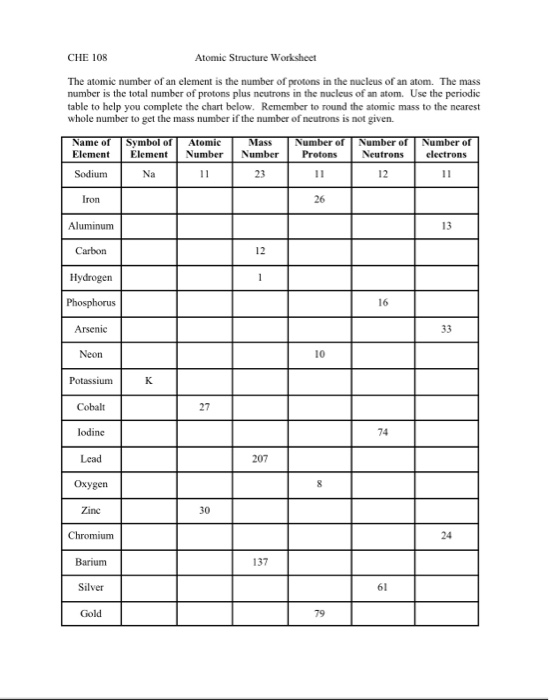
electron | Definition, Mass, & Facts | Britannica 22/09/2022 · electron, lightest stable subatomic particle known. It carries a negative charge of 1.602176634 × 10−19 coulomb, which is considered the basic unit of electric charge. The rest mass of the electron is 9.1093837015 × 10−31 kg, which is only 11,836the mass of a proton. An electron is therefore considered nearly massless in comparison with a proton or a neutron, … Worksheet Atomic Structure Answer Key - covid19.gov.gd Atomic Mass and Atomic Number Worksheet - Key Name of Element Symbol Atomic Number Atomic Mass Protons. Neutrons Electrons copper Cu 29 64 29 35 29 tin Sn 50 ... Atomic structure worksheet answers complete the chart Neutrons Mass Number Number of Electrons Uranium 238 8 8 hydrogen 1 82 207 Phosphorus 15 5. Worksheets - Answers. The atom is because this is also the ...
Atomic mass and atomic number worksheet answers. Worksheet Atomic Structure Answer Key - covid19.gov.gd Atomic Mass and Atomic Number Worksheet - Key Name of Element Symbol Atomic Number Atomic Mass Protons Neutrons Electrons copper Cu 29 64 29 35 29 tin Sn 50 119 ... › file › 12577534Basic Atomic Structure Worksheet ANSWERS - Course Hero Basic Atomic Structure Worksheet ANSWERS 1 a) protons b) neutrons c) electrons a) Positive b) Neutral c) negative 2 atomic number (or identity); charge 3 protons; electrons (in a neutral charge atom only!); same 4 average atomic weight; mass 5 mass number; nucleus 6 neutrons; protons (or atomic number); mass number 7 Lithium = Li = 3 Bromine ... Basic Atomic Structure Worksheet Key - Neshaminy School District have the atomic number. The mQSS of an element is the average mass of an element's naturally occurring atom, or isotopes, taking into account the Of each isotope. The q of an element is the total number of protons and neutrons in the of atom. The mass number is used to calculate the number of O in one atom of an element. In Isotope Worksheet Answer Key - ISD 622 mass # a35 33 # of protons 19 # of neutrons 22 Isoto e name uranium-235 uranium-23 8 boron- 10 boron-11 atomic # Phos hocus-33 15 Write the hyphen notation and the nuclide (nuclear) symbol for an isotope that has 17 protons, 17 electrons, and 20 neutrons. 37 Isotopes are atoms of the same element with a different number of
Mass Number and Isotopes Practice KEY Name: Key. Date: Period: 1-6. Atomic Mass and Atomic Number Worksheet mass number. Symbol. Atomic. Atomic. Protons Neutrons. Number. Mass. study.com › academy › practiceQuiz & Worksheet - Atomic Number and Mass Number | Study.com Print Atomic Number and Mass Number Worksheet 1. If Atom #1 has 19 protons and 22 neutrons, and Atom #2 has 20 protons and 22 neutrons, are these isotopes of the same element? en.wikipedia.org › wiki › Atomic_numberAtomic number - Wikipedia The atomic number or nuclear charge number (symbol Z) of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (n p) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements. phet.colorado.edu › sims › htmlIsotopes and Atomic Mass - PhET Isotopes and Atomic Mass - PhET
Chemistry: Atomic Number and Mass Number - The Wesley School Complete the following chart and answer the questions below. [Atomic #=# Protons]. P. N. Mass #. Element. Name. Atomic. Number. Number of. Protons. › cms › lib07Atomic Mass and Atomic Number Worksheet Key Atomic Mass and Atomic Number Worksheet - Key Name of Element Symbol Atomic Number Atomic Mass Protons Neutrons Electrons copper Cu 29 64 29 35 29 tin Sn 50 119 50 69 50 iodine I 53 127 53 74 53 uranium U 92 238 92 146 92 potassium K 19 39 19 20 19 lithium Li 3 7 3 4 3 oxygen O 8 16 8 8 8 gold Au 79 197 79 118 79 › cms › lib6Basic Atomic Structure Worksheet Key - Neshaminy School District have the atomic number. The mQSS of an element is the average mass of an element's naturally occurring atom, or isotopes, taking into account the Of each isotope. The q of an element is the total number of protons and neutrons in the of atom. The mass number is used to calculate the number of O in one atom of an element. In › molecular-mass-calculationsMolecular Mass Calculations - ThoughtCo Mar 11, 2019 · Find the atomic mass for each element by using the mass given in the Periodic Table. Multiply the subscript (number of atoms) times the atomic mass of that element and add the masses of all of the elements in the molecule to get the molecular mass. For example, multiple the subscript 12 times the atomic mass of carbon (C).
Atomic Mass and Atomic Number Worksheet Key - StuDocu Atomic mass and atomic number worksheet atomic mass and atomic number worksheet key name of element copper cu atomic number 29 sn 50 119 50 69 50 iodine 53 ...
Atomic structure worksheet answers complete the chart Neutrons Mass Number Number of Electrons Uranium 238 8 8 hydrogen 1 82 207 Phosphorus 15 5. Worksheets - Answers. The atom is because this is also the ...
Worksheet Atomic Structure Answer Key - covid19.gov.gd Atomic Mass and Atomic Number Worksheet - Key Name of Element Symbol Atomic Number Atomic Mass Protons. Neutrons Electrons copper Cu 29 64 29 35 29 tin Sn 50 ...
electron | Definition, Mass, & Facts | Britannica 22/09/2022 · electron, lightest stable subatomic particle known. It carries a negative charge of 1.602176634 × 10−19 coulomb, which is considered the basic unit of electric charge. The rest mass of the electron is 9.1093837015 × 10−31 kg, which is only 11,836the mass of a proton. An electron is therefore considered nearly massless in comparison with a proton or a neutron, …
























0 Response to "44 atomic mass and atomic number worksheet answers"
Post a Comment