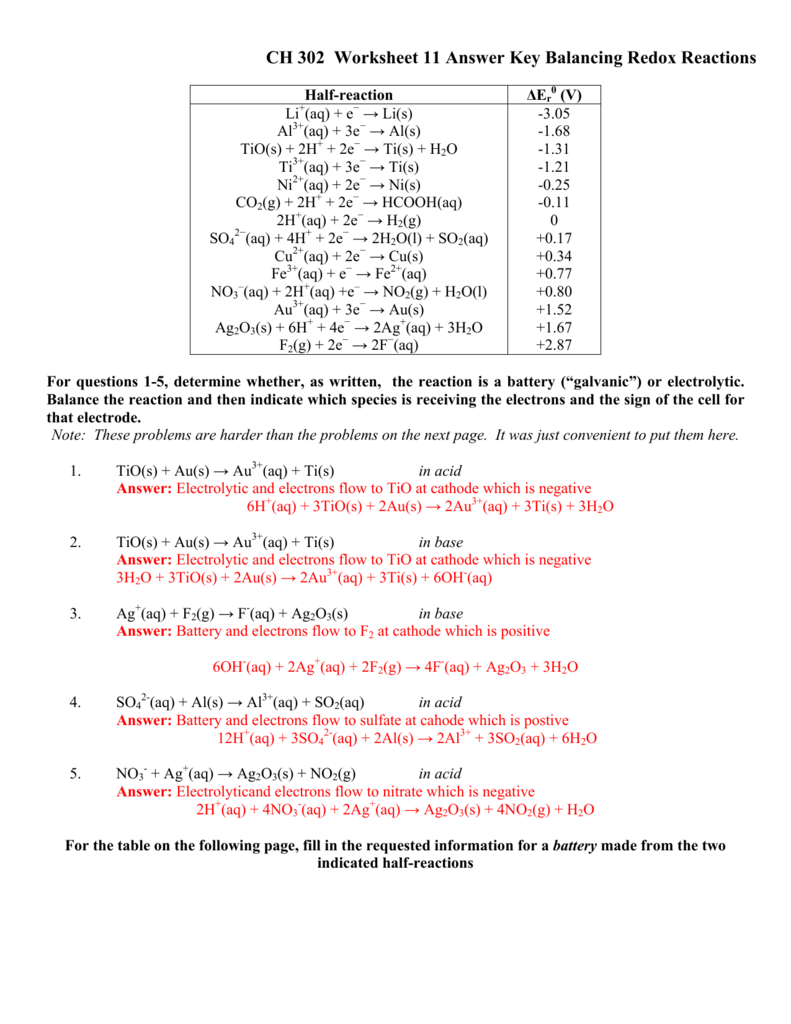
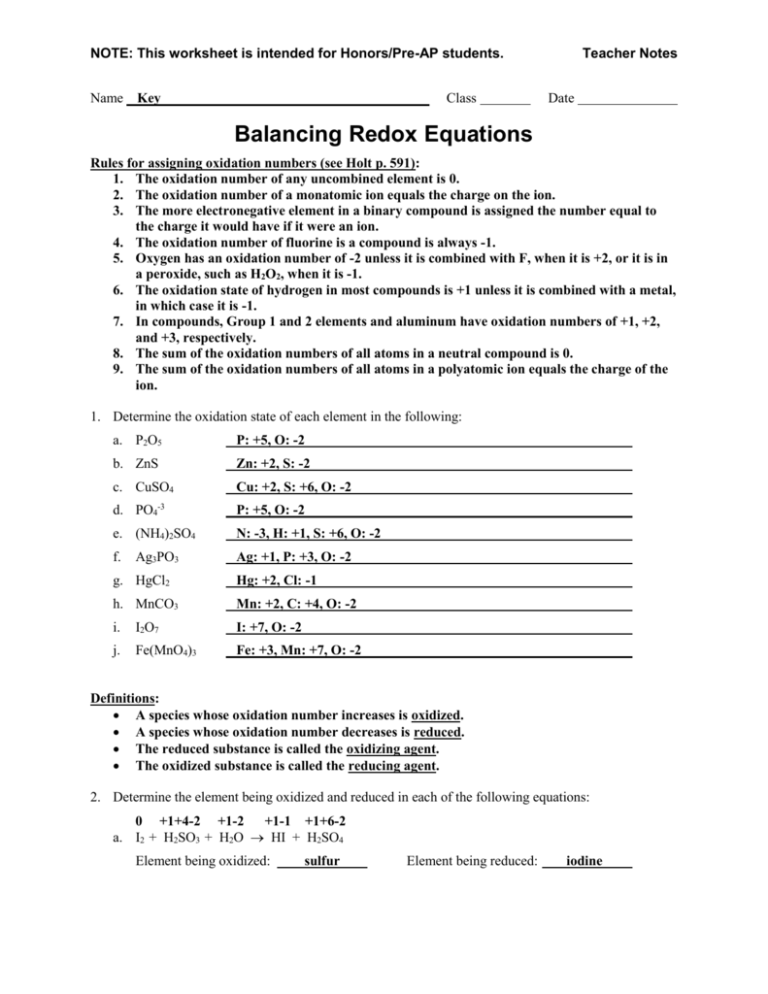
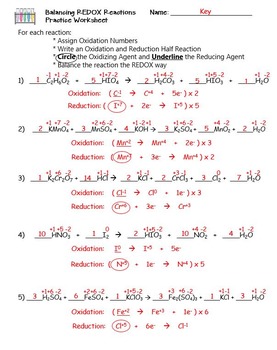
40 redox half reactions worksheet
Balancing Redox Reactions Oxidation/Reduction (Redox) reactions can be balanced using the oxidation state changes, as seen in the previous example. However, there is an easier method, which involves breaking a redox reaction into two half- reactions. This is best shown by working an example.
Chapter 20 Worksheet: Redox I. Determine what is oxidized and what is reduced in each reaction. Identify the oxidizing agent and the reducing agent, also. 1. 2Sr + O2 2SrO 2. 2Li + S Li2S 3. 2Cs + Br2 2CsBr 4. 3Mg + N2 Mg3N2 5. 4Fe + 3O2 2Fe2O3 6. Cl2 + 2NaBr 2NaCl + Br2 7. Si + 2F2 SiF4 8. 2Ca + O2 2CaO 9.
Write balanced equations for the following redox reactions: a. 2 NaBr + Cl 2 2 NaCl + Br 2 b. Fe 2 O 3 + 3 CO 2 Fe + 3 CO 2 in acidic solution c. 5 CO + I 2 O 5 5 CO 2 + I 2 in basic solution ; Write balanced equations for the following reactions: a. Cr(OH) 3 + Br 2 CrO 4 2-+ Br-in basic solution 10 OH-+ 2 Cr(OH) 3 + 3 Br 2 2 CrO 4 2-+ 8 H 2 O ...

Redox half reactions worksheet
How many electrons are transferred in the reaction as it is balanced? 2 e- For the following balanced redox reaction answer the following questions. 4NaOH(aq) + Ca(OH)2(aq) + C(s) + 4ClO2(g) ( 4NaClO2(aq) + CaCO3(s) + 3H2O(l) What is the oxidation state of Cl in ClO2(g)? 4 What is the oxidation state of C in C(s)? 0 What is the element that is ...
Balancing Redox Reactions: The Half-Reaction Method Balanced chemical equations accurately describe the quantities of reactants and products in chemical reactions. They serve as the basis of stoichiometry by showing how atoms and mass are conserved during reactions. Oxidation and reduction reactions need to be bal-
Worksheet # 5 Balancing Redox Reactions in Acid and Basic Solution Balance each half reaction in basic solution. 4. Cr 2O 7 2 - → Cr3+ 5. NO → NO 3-6. SO 4 2- → SO 2 7. MnO 2 → Mn 2O 3 Balance each redox reaction in acid solution using the half reaction method. 8. H 2O 2 + Cr 2O 7 2- → O 2 + Cr 3+ 9. TeO 3 2-+ N 2O 4 → Te + NO 3-10 ...
Redox half reactions worksheet.
Oxidation-Reduction Worksheet. For each reaction below, identify the atom oxidized, the atom reduced, the oxidizing agent, and the reducing agent. 1) Mg + 2HCl ( MgCl2 + H2. 2) 2Fe + 3V2O3 ( Fe2O3 + 6VO. 3) 2KMnO4 + 5KNO2 + 3H2SO4 ( 2MnSO4 + 3H2O + 5KNO3 + K2SO4.
Practice Problems: Redox Reactions. Determine the oxidation number of the elements in each of the following compounds: a. H 2 CO 3 b. N 2 c. Zn(OH) 4 2-d. NO 2-e. LiH f. Fe 3 O 4 Hint; Identify the species being oxidized and reduced in each of the following reactions: a. Cr + + Sn 4+ Cr 3+ + Sn 2+ b. 3 Hg 2+ + 2 Fe (s) 3 Hg 2 + 2 Fe 3+ c. 2 As ...
reaction and reduction half-reaction, identify the oxidizing and reducing agents and any spectator ions. Half - Reactions Homework ... Balance the following redox reactions by the half-reaction method, rewriting the balanced equations below the given unbalanced equation. Show your work below each reaction and
The half-reactions on a redox table are listed according to the relative strengths of the oxidizing and reducing agents, as illustrated by the sample table below. On the left side, the oxidizing agents are listed in order from weakest (at the top) to strongest (at ... Worksheet 1. The following reactions were performed. Construct a redox table.
The steps for balancing a redox reaction using the ion electron method are. 8 17 2008 1 57 32 pm. This worksheet shows you another method. Mno 2 mn 2o 3 balance each redox reaction in acid solution using the half reaction method. 1 break the equation into two half reactions one for the oxidation step. A trick to get around this is to balance ...
Redox Half Reactions and Reactions WS #1 Define each 1. loss of electrons 2. gain of electrons 3. oxidation by undergoing reduction 4. reduction by undergoing oxidation Write half reactions for Label each as oxidation or reduction. 5. 3e- oxidation 6.
Balancing Redox Reactions Worksheet 1 Balance each redox reaction in . acid. solution. Mn 2+ + BiO3 -Æ MnO4 -+ Bi 3+ MnO4 -+ S2O3 2- Æ S4O6 2- + Mn 2+
Predicting redox reactions using the half reaction table 1. Redox reactions worksheet. Cr 2o 7 2 cr3 5. Mn 2 bio3 æ mno4 bi 3 mno4 s2o3 2 æ s4o6 2 mn 2. In the reaction 2k cl2 2kcl the species. In the reaction al0 cr3 al3 cr0 the reducing agent is a. Ws 4 balancing redox reactions. 3mg n2 mg3n2 5. 2cs br2 2csbr 4. 5 2 customer reviews.
Worksheet 5 balancing redox reactions in acid and basic solution balance each half reaction in basic solution. 2 nabr cl 2 2 nacl br 2 b. B balance the oxygen atoms with h 2 o. No no 3 6. C balance the hydrogen atoms with h d in a basic medium add one oh to each side for every h step 4. Redox Reactions Exercise With Solutions.
Oxidation-Reduction Worksheet For each reaction below, identify the atom oxidized, the atom reduced, the oxidizing agent, the reducing agent, the oxidation half reaction, the reduction half reaction, and then balance the equation by the method of oxidation-reduction showing all electrons transfers.
(s) redox The two half reactions above also illustrate another important feature of balancing redox reactions. Notice that in each half reaction there is a balance both in the numbers of atoms of each kind and in the overall charge on each side. For example, in the oxidation Fe0 Fe3+ + 3e-
Worksheet Redox Half Reactions Practice With Answers. by Hedvig on December 5, 2021 December 5, 2021 Leave a Comment on Redox Half Reactions Practice With Answers. Ncert Solutions For Class 11 Chemistry Chapter 8 Redox Reactions Cbse Tuts Redoxreactionclass11 Redox Reactions 11th Chemistry Chemistry .
Balancing Redox Equations WorkSheet Oxidation Number Method for Balancing Redox Equations 1. 2. 3. 4. 5. 6. 7. Assign oxidation numbers to all elements and identify ...
Mno 2 mn 2o 3 balance each redox reaction in acid solution using the half reaction method. Redox reactions worksheet. No no 3 6. Cr 2o 7 2 cr3 5. Redox reactions 263 chemistry deals with varieties of matter and change of one kind of matter into the other. Transformation of matter from one kind into another occurs through the various types of ...
UNIT 6 - REDOX REACTIONS 6 • The oxidation number of an atom is the charge that would exist on an individual atom if the bonding were completely ionic • In simple ions, the oxidation number of the atom is the charge on the ion: - Na+, K+, H+ all have an oxidation number of +1 - Mg2+, Ca2+, Pb2+ all have an oxidation number of +2 - Cl-, Br-, I-all have an oxidation number of -1
Download printable Chemistry Class 11 Worksheets in pdf format, CBSE Class 11 Balancing of Redox Reactions Worksheet A has been prepared as per the latest syllabus and exam pattern issued by CBSE, NCERT and KVS. Also download free pdf Chemistry Class 11 Assignments and practice them daily to get better marks in tests and exams for Grade 11. Free chapter wise worksheets with answers have been ...
13.2 Writing redox and half-reactions (ESCQY) Redox reactions and half-reactions (ESCQZ) Remember from Grade 11 that oxidation and reduction occur simultaneously in a redox reaction. The reactions taking place in electrochemical cells are redox reactions. Two questions should be asked to determine if a reaction is a redox reaction:
The Scientific Classroom. 5. $1.20. PDF. REDOX: Writing Half Reactions Practice Worksheet Students will be practicing writing half reactions for a REDOX reaction by completing this practice worksheet. The worksheet contains 5 questions that require assigning oxidation numbers, writing an oxidation and reduction half reaction, and identify.
24. In the reaction Mg+Cl2!MgCl2, the correct half-reaction for the oxidation that occurs is A. Mg+2e !Mg2+ B. Cl2 +2e !2Cl C. Mg !Mg2+ +2e D. Cl2!2Cl +2e 25. The reaction that takes place in a chemical cell is best classi ed as A. fusion B. redox C. transmutation D. cracking 26. Which equation represents the half-reaction that takes place at ...
balance REDOX reactions well, you must first be able to assign oxidation numbers well. Oxidation - The loss of electrons, resulting in a more positively charged species. Reduction - The gain of electrons, resulting in a more negatively charged species. When presented with a REDOX reaction in this class, we will use the "half-reactions ...
Balancing Redox Equations Method 2: Half-reaction method 1. Divide the skeleton reaction into two half-reactions, each of which contains the oxidized and reduced forms of one of the species 2. Balance the atoms and charges in each half-reaction - Atoms are balanced in order: atoms other than O and H, then O, then H
Recognizing redox reactions worksheet answers. 3mg n2 mg3n2 5. Balance each of the following half cell reactions. Suited for student in y10 and y11. In the reaction mg cl2 mgcl2 the correct half reaction for the oxidation that occurs is a. Redox reactions are a chemical reaction in which electrons are exchanged through oxidation and reduction.
Balancing Redox Reactions Oxidation/Reduction (Redox) reactions can be balanced using the oxidation state changes, as seen in the previous example. However, there is an easier method, which involves breaking a redox reaction into two half- reactions. This is best shown by working an example.































0 Response to "40 redox half reactions worksheet"
Post a Comment