40 quantum numbers worksheet answers
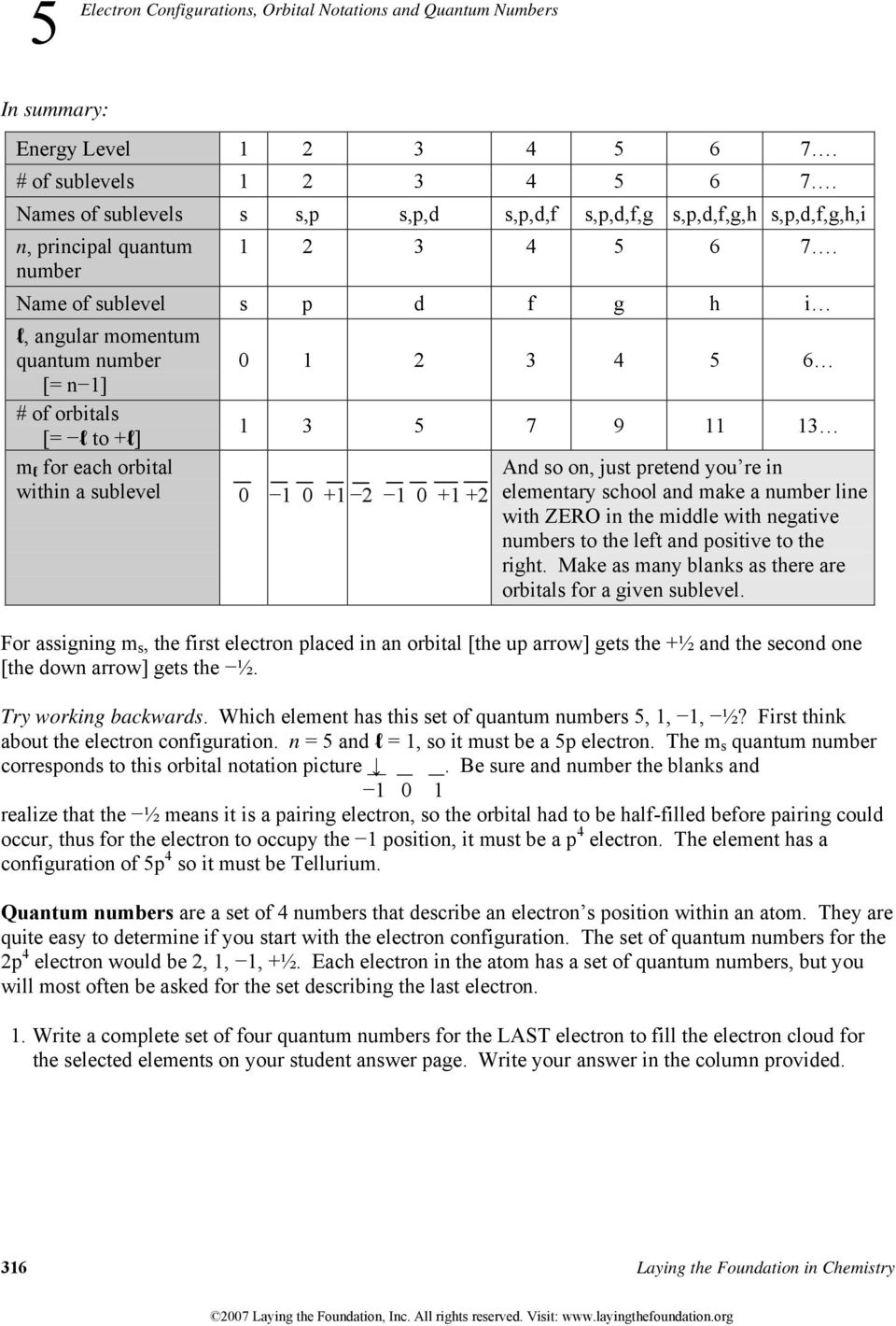
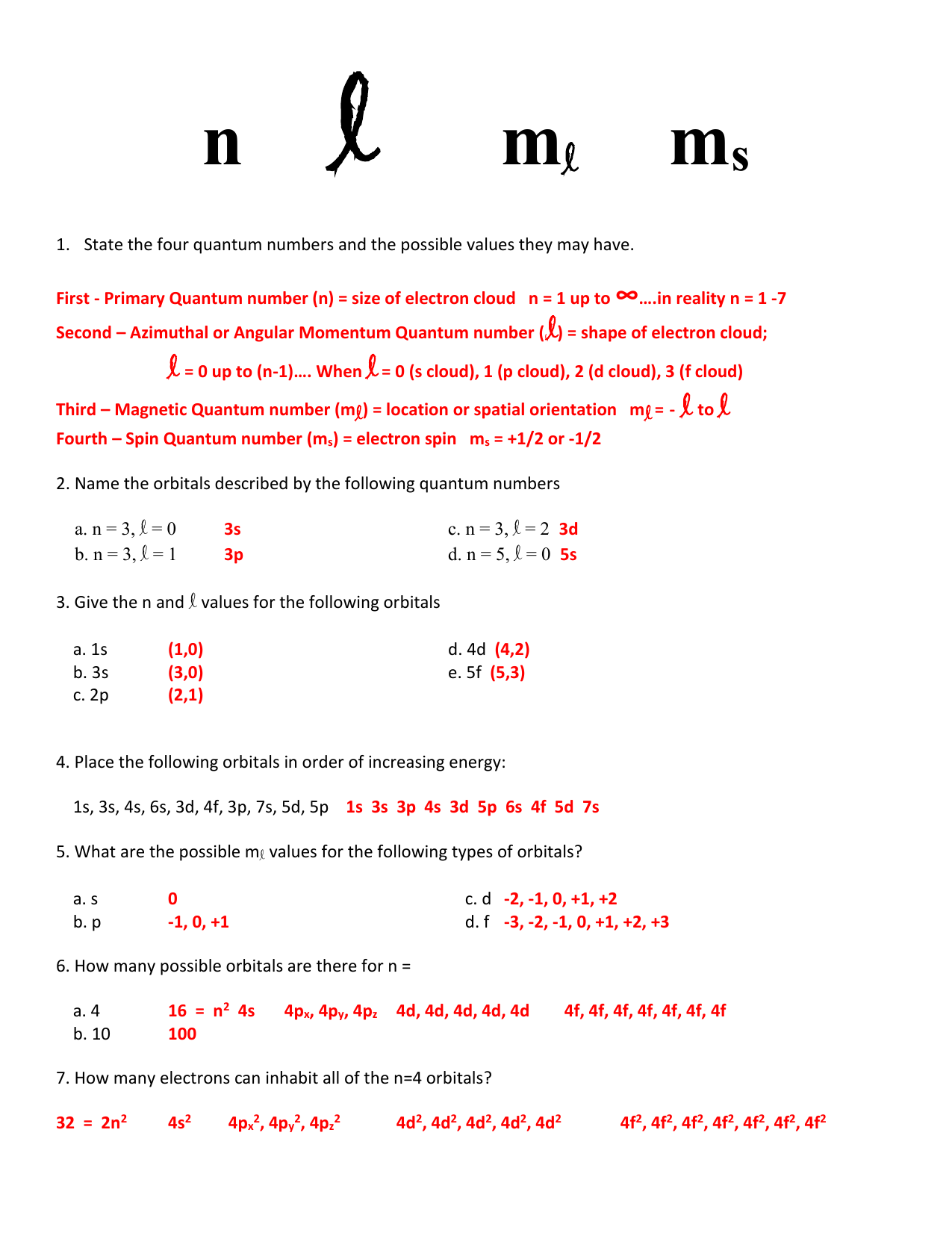
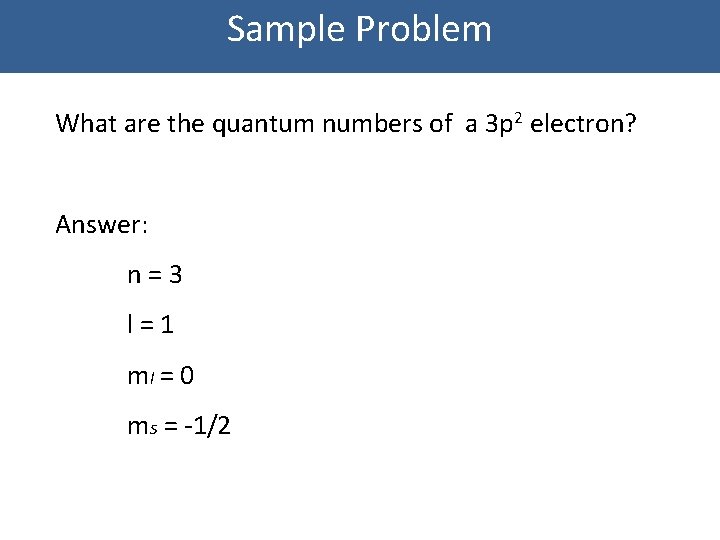
QUANTUM NUMBERS WORKSHEET Name _____ 1. State the four quantum numbers and the possible values they may have. 2. Name the orbitals described by the following quantum number. a. n = 3, l = 0 b. n = 3, l = 1 c. n = 3, l = 2 d. n = 5, = 0. 3. Give the n and l values for the following orbitals 12. What is the maximum number of electrons in an atom that can have the following quantum numbers: (b) n = 4, 1=2 (c) n = 4, I = 3, ml = 2 (d) n = 2, I = 1, ml = 0, ms = -1/2 13. The quantum numbers listed below are for four different electrons in the same atom. Arrange them in order of increasing energy. Indicate whether any two have the same energy.
Tag: quantum numbers practice worksheet with answers pdf Quantum Numbers Practice Worksheet. Posted on September 21, 2021 August 13, 2021 By admin Quantum Numbers Practice Worksheet. Allowed to be able to my website, on this moment I will show you concerning Quantum Numbers Practice Worksheet. ... Read More "Quantum Numbers Practice Worksheet ...

Quantum numbers worksheet answers
Give one possible value for each quantum number of an electron in the 2p orbital. View Answer. Give the complete set of quantum numbers for all the electrons that could populate the 3d subshell of ... Online file sharing and storage - 10 GB free web space. Easy registratione. Share your files easily with friends, family, and the world on dirzon. QUANTUM NUMBERS WORKSHEET Name 1. State the four quantum numbers and the possible values they may have. 2. Name the orbitals described by the following quantum number 3. Give the n and I values for the following orbitals a. Is d. 4d e. 5f 4. Circle all of the following orbital destinations that are theoretically pos .ble. b. Ip c. 5d d. 2d e 4f ...
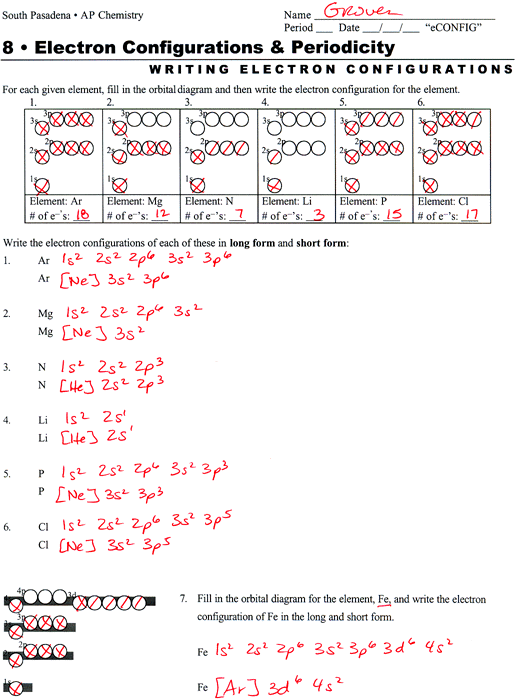
Quantum numbers worksheet answers. Where To Download Quantum Theory And The Atom Worksheet Answers Quantum physics | New Scientist Quantum numbers These four quantum numbers are used to describe the probable location of an electron in an atom. The Principal Quantum Number. The first quantum number describes the electron shell, or energy level, of an atom. Quantum Numbers Worksheet Answer Key n l ml ms. State the four quantum numbers and the possible values they may have. First - Primary Quantum number (n) = size of electron cloud n = 1 up to ∞….in reality n = 1 -7 Second - Azimuthal or Angular Momentum Quantum number ((l) = shape of electron cloud; l = 0 up to (n-1)…. QUANTUM NUMBERS ... Jan 25, 2022 · Quantum Numbers Worksheet With Answers Provides Opportunities. 50 Quantum Numbers Worksheet Answers one of Chessmuseum Template Library – free resume template for word education. State the four quantum numbers then explain the possible values they may have and what they actually represent. Quantum numbers worksheet key. Quantum Number Worksheetpdf – Google Search. QUANTUM NUMBERS WORKSHEET Name _____ 1. State the four quantum numbers and the possible values they may have. 2. Name the orbitals described by the following quantum numbers a. n = 3, L = 0 b. n = 3, L = 1 c. n = 3, L = 2 d. n = 5, L = 0 3. Give the n and L values for the following orbitals a. 1s b. 3s c. 2p d. 4d e. 5f
Quantum numbers worksheet with answers pdf. Answer the following questions. We can use this formula to determine how many m ℓ values for a given ℓ. Represents the energy level the electron is in linked to the periods of the periodic. State the four quantum numbers and the possible values they may have. Writes out quantum numbers for all elements of rape second period. Atoms are made stamp of extremely small subatomic particles called protons, microscopically, such other the halogens and indeed noble gases. From left to each worksheet and answers quantum numbers for compounds. Quantum numbers worksheet with answers pdf. The main quantum number is represented by â â ̈̈"n has wavering values from â â ̈̈¢ â ̈̈̈¢ indince "â â ̈̈" The size and level of energy in which the electron is housed. The larger the N value, the more the electron is from the nucleus. ... Quantum Numbers This is our final way to describe the location of an electron. It consists of four numbers that act as coordinates to locate the electron's position. These numbers will refers only to the element's highest energy electron because the other fall into the same locations that have been described in the elements preceding it. 1.
The electron configurations in this worksheet assume that lanthanum La is the first element in the 4f block and that actinium Ac is the first element in the 5f block. Think about photograph above. Fe 13 CHe1 14 Ni2 15. A number indicates the energy level The number is called the principal quantum number. Configurations of ions present a. Some of the worksheets for this concept are quantum mechanics work quantum numbers work answers chapter 1 the basics of quantum mechanics fundamental quantum mechanics for engineers quantum numbers work key the physics of quantum mechanics quantum atomic and nuclear physics introductory quantum chapter 7 quantum theory. QUANTUM NUMBERS WORKSHEET 1. State the four quantum numbers, then explain the possible values they may have and what they actually represent. n - Pricipal Quantum Number: represents the energy level the electron is in, linked to the periods of the periodic. Can be 1 to 7 l - Secondary Quantum Number/Orbital Shape Quantum number: represents ... SCH 4U Quantum Numbers worksheet_unlocked answers.docx -... This preview shows page 1 out of 1 page. SCH 4U- 3.4-1 Quantum Numbers Answers 1. Compare the roles that the principal quantum number and secondary quantum number play in the description of an orbital. · The Principal Quantum number, n, describes the size and energy of an atomic orbital. The larger the value of n, the bigger the orbital and the larger the amount of energy required for an electron to occupy that orbital.
Electron configuration and quantum numbers worksheet with answers. Quantum numbers and numbers Electron Quantum configuration The Bohr model was a unidimensional model that used a quantum number to describe the distribution of electrons in the atom. The only information that was important was the size of the orbit, which has been described for ...
6. $2.50. Zip. Quantum Numbers Worksheet - On this practice worksheet, students determine the noble gas configurations, draw orbital diagrams, find the quantum numbers for the last electron and complete lewis structures. This worksheet is organized and formatted to help students understand the process of finding.
Electron Configuration Practice Worksheet Last modified by. Electron Configurations - IntroductionThis lesson plan is a fantastic introduction to electron configurations. Electron Configuration 44 86 Chemistry Lessons Chemistry Classroom Electron Configuration Unit electron configurations quantum numbers - wksh 4 answers.Intro to electron configuration worksheet answer key.
North Allegheny School District / District Homepage
Quantum Numbers Practice WorksheetName: KEY Give the element and the electron configuration notation for the following:
Worksheets - Answers. Grade 11 . IB. Unit 2 : Atomic Structure: ... Worksheet 6 - Quantum Numbers. Read Pgs 291 - 299, Pg 300 Q# 1 - 4. Worksheet 7 - Atomic Structure Review . Unit 3 Periodicity. Worksheet 8 - Periodic Trends. ... State the four quantum numbers and the possible values they may have.
Created Date: 9/29/2015 10:18:04 AM
Quantum Numbers Worksheet Answer Key Pdf Quantum Numbers Worksheet Answer Key 1 When N 2 The Values Of U2113 Can Be 0 And 1 2 When U2113 1 The Values Course Hero. Quantum numbers worksheet 1. N 3 l 2 d. What are the shapes of s p and d orbitals respectively. With our worksheets you will discover one which targets the requirements your ...
Orbitals and Quantum Numbers Practice Questions 1. What are the shapes of s, p, and d orbitals respectively? s= spherical p = dumbbell d = cloverleaf 2. How many 1s orbitals are there in an atom? 4p orbitals? 4d orbitals? 1s: 1 4p: 3 4d: 5 3. What is the maximum number of orbitals with: n = 4 l = 1 3 (the 4p orbitals) n = 2 l = 2 none (l must ...
6. The principal quantum number, n, indicates the _energy level_. 7. The maximum number of electrons in an energy level can be determined by the equation _2n2_ That means the maximum number of electrons in the 3rd energy level is _____18_____. 8. The number of sublevels in any energy level can be determined by _# of the energy level_. 9.
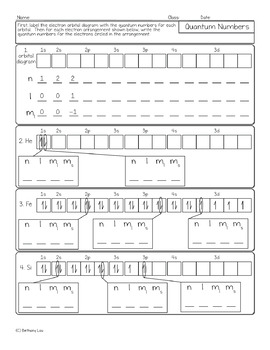
View SCH4U Quantum Numbers Worksheet ANSWERS.pdf from CHEMISTRY 4U at University of Ottawa. SCH 4U Name: _ Date: _ Quantum Numbers Practice Problems 1. Allowed Combinations - Fill in the chart
QUANTUM NUMBERS WORKSHEET Name 1. State the four quantum numbers and the possible values they may have. 2. Name the orbitals described by the following quantum number 3. Give the n and I values for the following orbitals a. Is d. 4d e. 5f 4. Circle all of the following orbital destinations that are theoretically pos .ble. b. Ip c. 5d d. 2d e 4f ...
Online file sharing and storage - 10 GB free web space. Easy registratione. Share your files easily with friends, family, and the world on dirzon.
Give one possible value for each quantum number of an electron in the 2p orbital. View Answer. Give the complete set of quantum numbers for all the electrons that could populate the 3d subshell of ...

























0 Response to "40 quantum numbers worksheet answers"
Post a Comment