43 average atomic mass worksheet
PDF Average atomic mass worksheet answers with work - Florent MAUSSION Average atomic mass worksheet answers with work The element bromine has three naturally-occurring isotopes. A mass spectrum of molecular Br2 shows three peaks with mass numbers of 158 u, 160 u, and 162 u. Use this information to determine which isotopes of Br occur in nature. 79 u, 81 u Calculate the elemental atomic mass of Mg if the naturally ... Molecular Mass Calculations - ThoughtCo 11.03.2019 · Note About Molecular Mass and Isotopes . The molecular mass calculations made using the atomic masses on the periodic table apply for general calculations, but aren't accurate when known isotopes of atoms are present in a compound. This is because the periodic table lists values that are a weighted average of the mass of all natural isotopes of each element.
Chemistry Quizzes | Study.com Interested in seeing how well you know a particular chemistry concept? Take Study.com's brief multiple-choice quiz. Obtain rapid feedback and results to understand how well you performed. The quiz ...

Average atomic mass worksheet
PDF Average Atomic Mass Worksheet - Mr. Macha's Class Website Average Atomic Mass Worksheet Calculate the average atomic masses. Show your work! Round all answers to two decimal places. 1. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? 2. Average Atomic Mass Activity Teaching Resources | TpT This is a self-paced lesson for students to learn about how isotopes are used to calculate the average atomic mass shown on the periodic table.Slides 2-5 shows students how to use periodic table for the number of protons and how to use hyphen and isotope notation. Quiz & Worksheet - Average Atomic Mass | Study.com About This Quiz & Worksheet. Test your understanding of average atomic mass and the steps used to calculate it with this quiz/worksheet combo. All of the questions on these resources are multiple ...
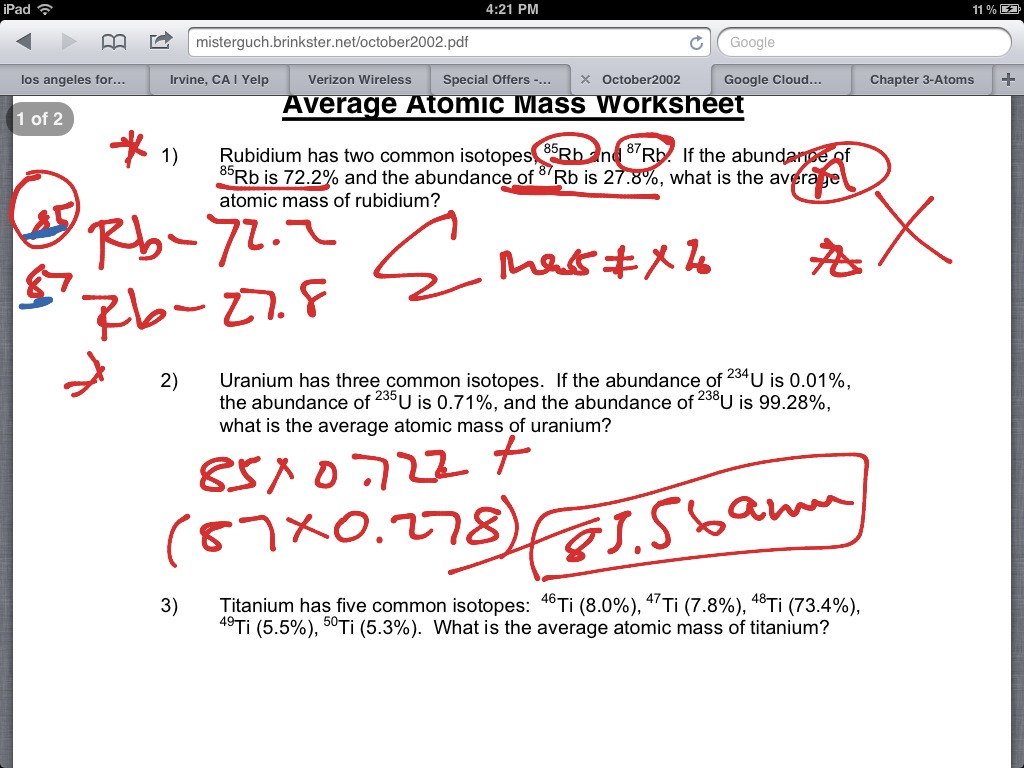
Average atomic mass worksheet. Year 10 Science Worksheets - Key Stage 4 - EdPlace Calculate Relative Atomic Mass and Relative Formula Mass TRY WORKSHEET FOR FREE. ... *Student progress - Students who used EdPlace learning materials progressed by an average of 153% in English, maths and science over an academic year. This was analysed using the scores from the EdPlace database with all activities taken by students managed by parent accounts … Quiz & Worksheet - Atomic Number and Mass Number | Study.com About This Quiz & Worksheet. There's quite a lot to be learned about atoms. This article and quiz focuses on just some of that, by asking you to recall facts about the atomic number and mass number. Average Atomic Mass Worksheet.docx - Course Hero Average Atomic Mass Worksheet: show all work. 1) Rubidium is a soft, silvery-white metal that has two common isotopes,85Rb and87Rb. If the abundance of85Rb is 72.2% and the abundance of87Rb is 27.8%, what is the average atomic mass of rubidium? 2) Uranium is used in nuclear reactors and is a rare element on earth. Uranium has three common isotopes. PDF Atoms and Isotopes Worksheet Atoms and Isotopes Worksheet 1. Fill in the table with the correct information. Isotope Isotope Notation Atomic # Protons Electrons Neutrons Oxygen-16 Bromine-80 ... Calculate the average atomic mass of chlorine if its isotopes and % abundance are as follows. Show work. Mass of Isotope % abundance 36.96590 24.47 (.2447)(36.96590) = 9.04556 ...
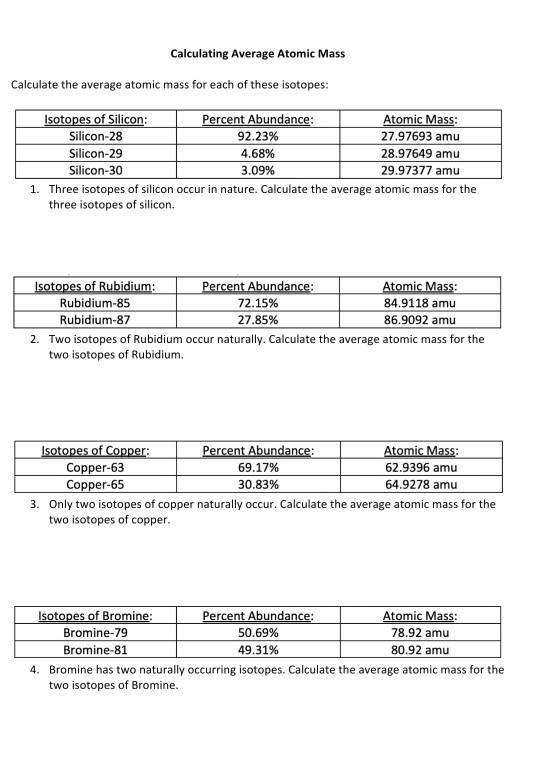
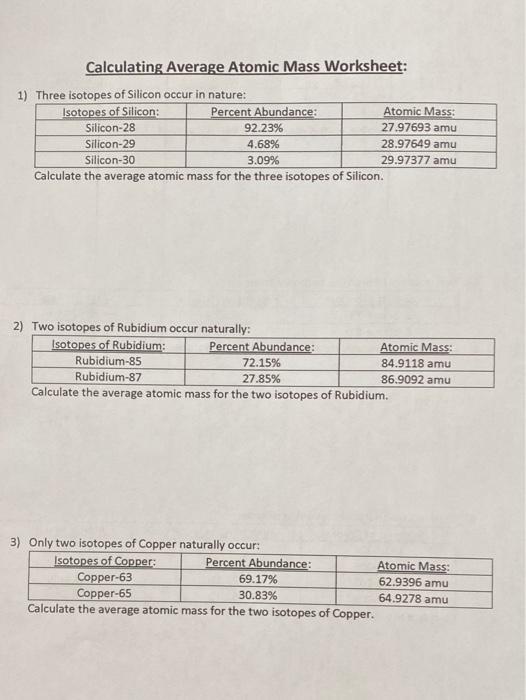
PDF Average Atomic Mass Practice Problems - scott.kyschools.us 1. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? 2. Calculate the average atomic mass of lithium, which occurs as two isotopes that have the following atomic masses and abundances in nature: 6.017 u, 7.30% and PDF Average Atomic Mass Worksheet Key - University of South Florida Average Atomic Mass Worksheet Key Directions Solve each of the following. Obey the rules of significant digits. 1. Due to its strength and lightweight, magnesium is often used in the construction of racing cars ... average atomic mass. If the relative abundance of hydrogen-1 is 99.5% and a sample of PDF Calculating Average Atomic Mass Worksheet - SI PROGRAM Calculating Average Atomic Mass Worksheet: 1) Three isotopes of Silicon occur in nature: Atomic Mass: 27.97693 amu 28.97649 amu 29.97377 amu Isoto es of Silicon: Silicon-28 Silicon-29 Silicon-30 Percent Abundance: 92.23% 4.68% 3.09% Calculate the average atomic mass for the three isotopes of Silicon. (-9 Y(.o ( zq.q 7377) 0/0 Chemistry: Average Atomic Mass Worksheet Flashcards | Quizlet Calculate the average atomic mass for each element based on the natural abundance of its isotopes. Terms in this set (4) Find the average atomic mass for Li if 7.5% of Li atoms are 6Li with a mass of 6.0151223 amu and 92.5% are 7Li with a mass of 7.0160041 amu. (7.5 x 6.01251223) + (92.5 x 7.0160041) / 100; 45.0938417 + 648.9803793 / 100 =
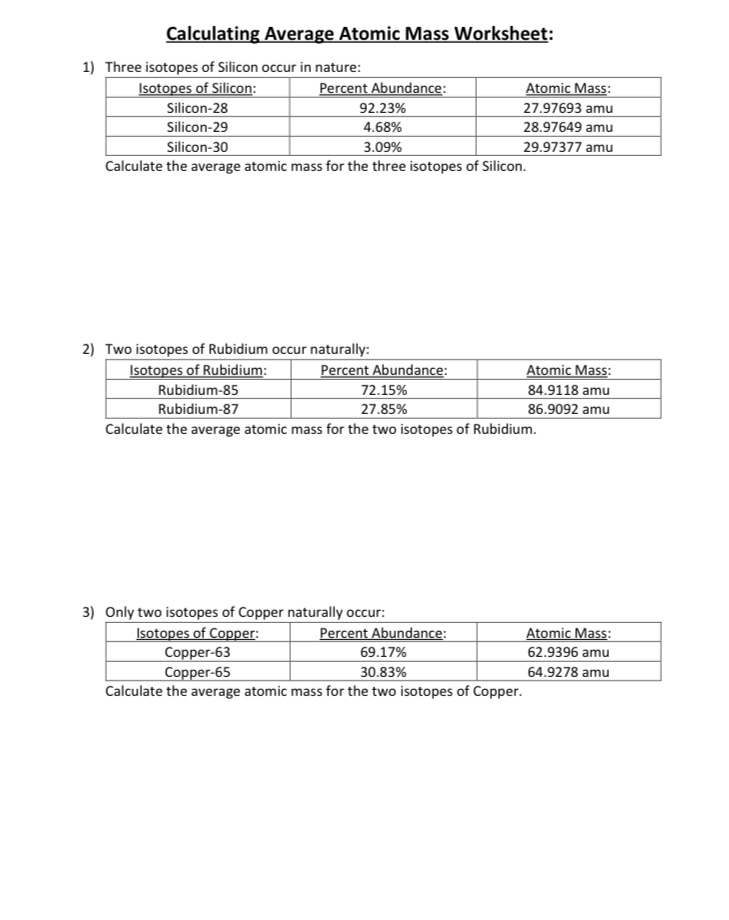
PDF NAME Average Atomic Mass Worksheet: show all work. Average Atomic Mass Worksheet: show all work. 1) Rubidium is a soft, silvery-white metal that has two common isotopes, 85Rb and 87Rb. If ... The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu. 65Cu = 64.9278 amu 7) Magnesium consists of three naturally occurring isotopes. ... DOC Calculating Average Atomic Mass Worksheet Name______________________ Calculating Average Atomic Mass Worksheet Name______________________ Percents need to be in decimal form 1. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are 69.2% and 30.8% respectively. Calculate the average atomic mass of copper. 2. PDF isotopic abundance practice problems - CHEMISTRY name: !suggested answers date: _____ ! isotopic abundance - practice problems The atomic mass for each element appearing on the periodic table represents the weighted average of masses for each individual isotope of an element. For example, the atomic mass of carbon is reported as 12.011 amu (atomic mass units). Carbon is composed primarily of two isotopes; carbon-12 and carbon-14. DOC Calculating Average Atomic Mass Worksheet Name______________________ Calculate the average atomic mass of copper. 3. Calculate the average atomic mass of sulfur if 95.00% of all sulfur atoms have a mass of 31.972 u, 0.76% has a mass of 32.971u and 4.22% have a mass of 33.967u. 4. Naturally occurring strontium consists of four isotopes, Sr-84, Sr-86, Sr-87 and Sr-88. Below is the data concerning strontium:
Calculating Average Atomic Mass Worksheet - Name ___Lisa ... - StuDocu The relative abundance and atomic masses are: 69% for mass of 62 30% for mass of 64. Calculate the average atomic mass of Copper. Show ALL work for full credit. 69/100 x 62 = 43. 30/100 x 64 = 19. ggghvhvhvhvhhv= 63-----Calculate the average atomic mass of Sulfur if: 95% of all Sulfur atoms have a mass of 31 u, 0% has a mass of 32 and 4% have a ...
PDF Worksheet #1 Average Atomic Mass Worksheet #1 Name _____ Average Atomic Mass Use the following data to determine the average atomic mass of each element. Please show all calculations. Round your answers to three decimal places in this activity and box your answer. ... Atomic Mass (amu) Chlorine-35 75.78 34.9688 Chlorine-37 24.22 36.9659 . Author: Sue Lowder Created Date:
8th Grade Free Science worksheets, Games and Quizzes Matter and Mass. Mass Continuity Formula. Density Formula. Volume Continuity Formula. Density Facts. Density Examples. Force and Motion . Physical Science : Velocity Practice Problems Quiz. Kinematics : Speed and Velocity Quiz. Velocity Formula. Distance Speed Time Formula. Speed vs. Velocity. Average Velocity Formula (displacement over time) Physical and Chemical change. …
Average Atomic Mass Practice Problems Quiz - Quizizz Calculate the average atomic mass of an element with the follow isotope information: 4.35% have a mass of 49.9461 amu, 83.79% have amass of 51.9405 amu, 9.50% have a mass of 52.9407 amu, and 2.36% have a mass of 53.9389 amu. answer choices 51.99 amu 52.19 amu 53.45 amu 17.33 amu Question 4 900 seconds
DOC Chemistry Worksheet - Livingston Public Schools 72.6amu Complete the chart below for the element silicon, which has three naturally occurring isotopes: Isotope Atomic mass (amu) Natural abundance (atom %) 28Si 28.0 92.2 29Si 29.0 ? 30Si ?? 3.1 The average atomic mass of silicon is 28.09amu. %29Si = 4.7% mass = 29.4amu Calculate the relative abundance of each isotope of iridium.
Worked Chemistry Problem Examples - ThoughtCo 22.11.2019 · Alphabetical Index of Chemistry Problem Types . Included in this list are printable pdf chemistry worksheets so you can practice problems and then check your answers. You may also browse chemistry problems according to the type of problem.
Atomic Mass Atomic Number Worksheets - K12 Workbook *Click on Open button to open and print to worksheet. 1. Chemistry Worksheet, Atomic Number and Mass Number 2. Atomic Structure Review Worksheet Answers 3. Mayfield High School 4. Chemistry Atomic Number And Mass Number 5. Atomic Structure Periodic Table Worksheet Answers 6. CHAPTER 4: Chemical patterns Worksheet 4.2 Science Quest ... 7.
Lesson Worksheet:Percentage Isotopic Abundance | Nagwa In this worksheet, we will practice calculating percentage isotopic abundances from the relative atomic mass and isotopic masses. Q1: Chlorine has two stable isotopes, 3 5 C l and 3 7 C l , with atomic masses 34.9689 u and 36.9659 u respectively. The relative abundance of 3 7 C l in an average sample of chlorine is 3 7 3 5 C l C l = 0. 3 1 9 6.
Isotopes & Relative Atomic Mass (solutions, examples, videos) Atomic Mass Units. Average Atomic Mass and definition of atomic mass unit. Show Video Lesson. Relative atomic mass. The relative atomic mass (A r) of an element is the average mass of the naturally occurring atoms of the element. This quantity takes into account the percentage abundance of all the isotopes of an element which exist. The formula for relative atomic mass …
Atomic Mass Worksheet | Teachers Pay Teachers Calculating Average Atomic Mass Worksheet Distance Learning. by. Mr Connors Laboratory. $2.00. PDF. This smart sheet serves as a followup to my Average Atomic Mass Phet Lab. It has 7 questions requiring the students to calculate Average Atomic Mass and the Percent Abundance of the elements.
average atomic mass worksheets average atomic mass interactive and downloadable worksheets. Search results: average atomic mass
Build an Atom - Atoms | Atomic Structure | Isotope Symbols Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas!
Average Atomic Mass POGIL (Key).pdf - Google Docs Average Atomic Mass POGIL (Key).pdf. Average Atomic Mass POGIL (Key).pdf. Sign In ...
Dimensional analysis worksheet -Free PDF - Physicscatalyst This page contains Dimensional analysis worksheet for class 11 along with downloadable pdf. Practice these problems for better understanding of this topic.
Basic Atomic Structure Worksheet Key - Neshaminy School District in a neutral atom of that element. The atomic number gives the "identity" of an element as well as its location on the periodic table. No two different elements will have the atomic number. The mQSS of an element is the average mass of an element's naturally occurring atom, or isotopes, taking into account the Of each isotope.
Average Atomic Mass Worksheet-1.pdf - Average Atomic Mass... The average atomic mass between these two isotopes is 63.55 amu. Calculate the actual atomic mass of 65Cu. 7) Magnesium consists of three naturally occurring isotopes. The percent abundance of these isotopes is as follows:24Mg (78.70%),25Mg (10.13%), and26Mg (11.7%). The average atomic mass of the three isotopes is 24.31 amu.
PPT Calculating Average Atomic Mass - wsfcs.k12.nc.us Calculating Average Atomic Mass Unit 9 worksheet 2 Average atomic mass The decimal number on the periodic table The weighted average of all the isotopes of an element Depends on the percent (relative) abundance and the mass of each isotope Measured in "atomic mass units" (amu) Problem 1 Given: Element X has 2 isotopes Mass = 6 amu and percent abundance = 7.5% (0.075) Mass = 7 amu and ...
average atomic mass calculations worksheets average atomic mass calculations interactive and downloadable worksheets.
PDF Isotopes Average Atomic Mass - nyostrander.us 6. Weighted average of naturally occurring isotopes atomic mass 7. Total number of protons plus neutrons mass number 8. All electrons are in the lowest energy levels ground state 9. 1/12 the mass of a carbon-12 atom atomic mass unit (u) 10. How to solve for the number of neutrons mass number - atomic number 11.
Calculating Average Atomic Mass Worksheet - Name ... - StuDocu The relative abundance and atomic masses are: 69% for mass of 62 30% for mass of 64. Calculate the average atomic mass of Copper. Show ALL work for full credit.-----Calculate the average atomic mass of Sulfur if: 95% of all Sulfur atoms have a mass of 31 u, 0% has a mass of 32 and 4% have a mass of 33. Show ALL work for full credit.
PDF Practice Problems - Denton ISD 37. Calculate the average atomic mass of chlorine. 2. Copper has two isotopes. Copper-63, which has an atomic mass of 62.93 u and copper-65, which has an atomic mass of 64.93 u. In any sample of copper atoms, 69.1% will be copper-63 and 30.9% will be copper-65. Calculate the average atomic mass of naturally occurring copper. 3. One atom has 20 ...
How to Calculate Average Atomic Mass | Chemistry | Study.com Calculate the average atomic mass of boron given that 19.8% of its naturally occurring atoms have a mass of 10.013 amu and 80.2% have a mass of 11.009 amu. ... Quiz & Worksheet - Iceberg Model in ...
Quiz & Worksheet - Average Atomic Mass | Study.com About This Quiz & Worksheet. Test your understanding of average atomic mass and the steps used to calculate it with this quiz/worksheet combo. All of the questions on these resources are multiple ...
Average Atomic Mass Activity Teaching Resources | TpT This is a self-paced lesson for students to learn about how isotopes are used to calculate the average atomic mass shown on the periodic table.Slides 2-5 shows students how to use periodic table for the number of protons and how to use hyphen and isotope notation.
PDF Average Atomic Mass Worksheet - Mr. Macha's Class Website Average Atomic Mass Worksheet Calculate the average atomic masses. Show your work! Round all answers to two decimal places. 1. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? 2.


![10+Average+Atomic+Mass-S[1].pdf - Average Atomic Mass How are ...](https://www.coursehero.com/thumb/c5/c2/c5c28339a578af1997f661bdcd75517225f40678_180.jpg)
























0 Response to "43 average atomic mass worksheet"
Post a Comment