39 atoms ions isotopes worksheet answers
Join LiveJournal WebPassword requirements: 6 to 30 characters long; ASCII characters only (characters found on a standard US keyboard); must contain at least 4 different symbols; Kahoot WebHier sollte eine Beschreibung angezeigt werden, diese Seite lässt dies jedoch nicht zu.
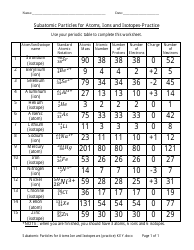
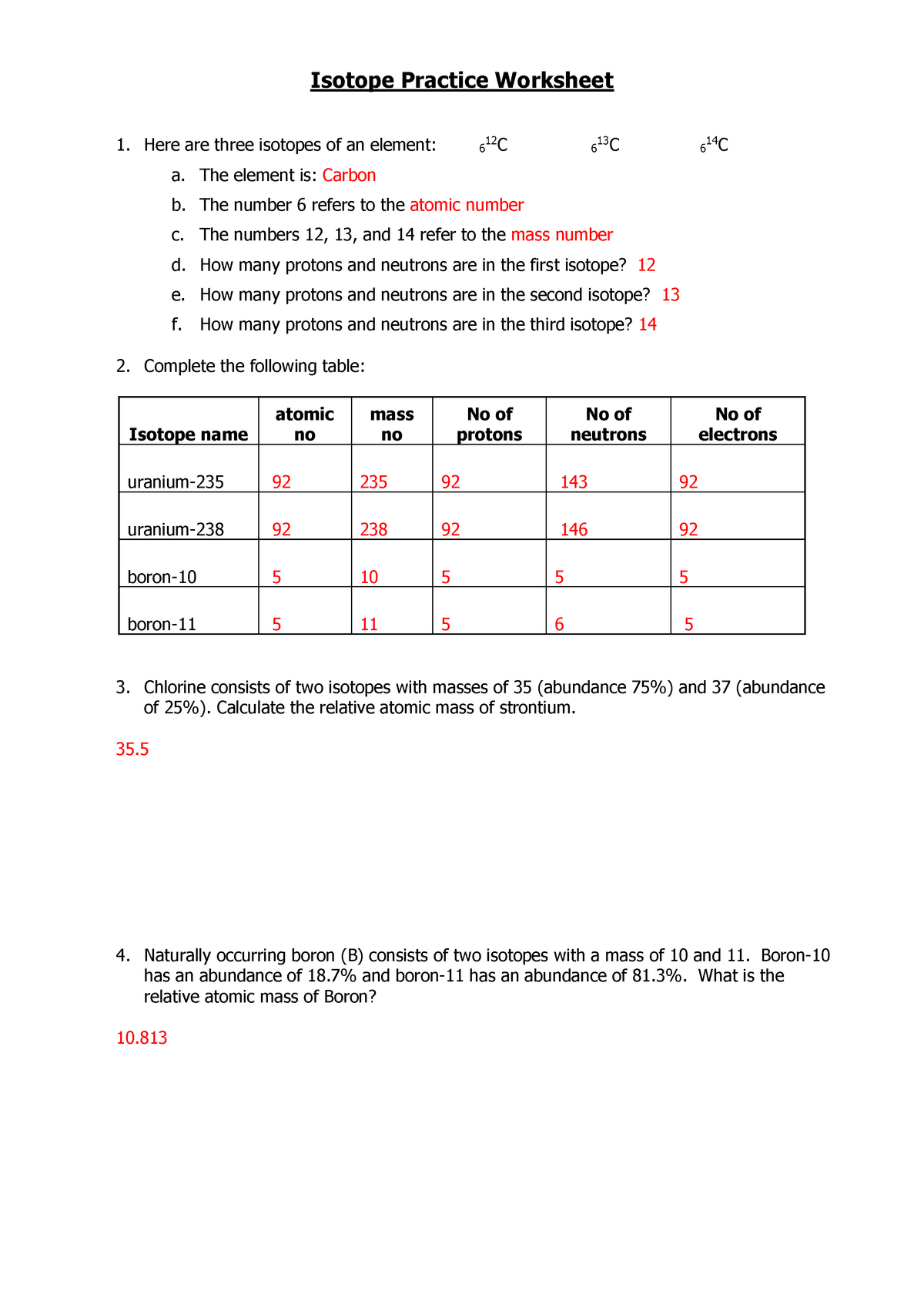
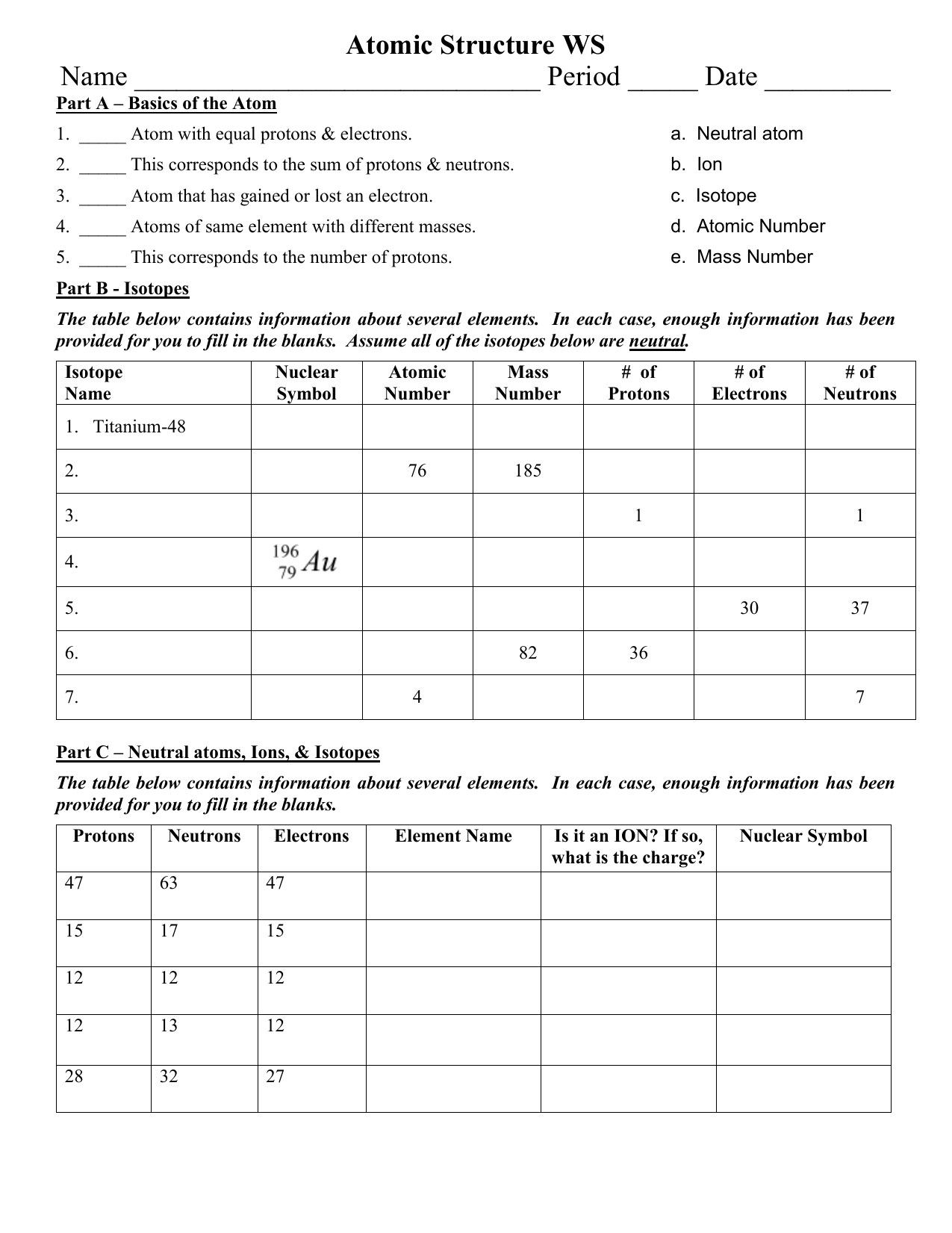
Atomic Structure Worksheet - Washoe County School District WebTheof an element is the average mass of an element 's naturally occurring atoms, or isotopes, taking into account theof each isotope. In order to calculate the number of neutrons you must subtract the from the. Give the symbol and number of protons in one atom of: Lithium Mercury Iron. Com lete the table below. Symbol Atomic Number Mass …
Atoms ions isotopes worksheet answers
Chemistry 105 Exam 1 Flashcards | Quizlet (a) How many gold atoms are in a cube of this gold isotope which is 10.0 cm on each side if the density of gold is 19.3 g/cm3? Report your answer in scientific notation—and remember, you won't be reminded in future that all answers are to be reported with the correct significant digits. (b) How many electrons are in the cube? Chemistry Quizzes | Study.com Isotopes: Quiz & Worksheet for Kids . View Quiz. History of Atoms: Quiz & Worksheet for Kids . View Quiz. Analytical Chemistry Tools . ... Lewis Dot Structures of Polyatomic Ions . View Quiz ... Atoms, elements, molecules, compounds and mixtures Web4.1.1 A simple model of the atom, symbols, relative atomic mass, electronic charge and isotopes. 4.1.1.2 Mixtures. A mixture consists of two or more elements or compounds not chemically combined together. 4.1.1.1 Atoms, elements and compounds. All substances are made of atoms. An atom is the smallest part of an element that can exist.
Atoms ions isotopes worksheet answers. Success Essays - Assisting students with assignments online WebSuccess Essays essays are NOT intended to be forwarded as finalized work as it is only strictly meant to be used for research and study purposes. Writing & Naming Acids Video Notes worksheet ID: 3250349 Language: English School subject: Chemistry Grade/level: 9th through 12th grade Age: 14+ Main content: Naming acids, Polyatomic ions, negative ion Other contents: Add to my workbooks (0) Lifestyle | Daily Life | News | The Sydney Morning Herald WebThe latest Lifestyle | Daily Life news, tips, opinion and advice from The Sydney Morning Herald covering life and relationships, beauty, fashion, health & wellbeing 7.4 Formal Charges and Resonance - Chemistry 2e | OpenStax All oxygen atoms, however, are equivalent, and the double bond could form from any one of the three atoms. This gives rise to three resonance forms of the carbonate ion. Because we can write three identical resonance structures, we know that the actual arrangement of electrons in the carbonate ion is the average of the three structures.
Atomic number - Wikipedia The atomic number or nuclear charge number (symbol Z) of a chemical element is the charge number of an atomic nucleus.For ordinary nuclei, this is equal to the proton number (n p) or the number of protons found in the nucleus of every atom of that element. Build an Atom - Atoms | Atomic Structure | Isotope Symbols WebBuild an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! 2.3: Calculating Atomic Masses (Problems) - Chemistry LibreTexts WebPROBLEM \(\PageIndex{4}\) Average atomic masses listed by IUPAC are based on a study of experimental results. Bromine has two isotopes, 79 Br and 81 Br, whose masses (78.9183 and 80.9163 amu) and abundances (50.69% and 49.31%) were determined in earlier experiments. Calculate the average atomic mass of Br based on these experiments. Achiever Papers - We help students improve their academic … WebAll our academic papers are written from scratch. All our clients are privileged to have all their academic papers written from scratch. These papers are also written according to your lecturer’s instructions and thus minimizing any chances of plagiarism.
Chemistry - Wikipedia WebChemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds composed of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances.. In the scope of its … Atoms, elements, molecules, compounds and mixtures Web4.1.1 A simple model of the atom, symbols, relative atomic mass, electronic charge and isotopes. 4.1.1.2 Mixtures. A mixture consists of two or more elements or compounds not chemically combined together. 4.1.1.1 Atoms, elements and compounds. All substances are made of atoms. An atom is the smallest part of an element that can exist. Chemistry Quizzes | Study.com Isotopes: Quiz & Worksheet for Kids . View Quiz. History of Atoms: Quiz & Worksheet for Kids . View Quiz. Analytical Chemistry Tools . ... Lewis Dot Structures of Polyatomic Ions . View Quiz ... Chemistry 105 Exam 1 Flashcards | Quizlet (a) How many gold atoms are in a cube of this gold isotope which is 10.0 cm on each side if the density of gold is 19.3 g/cm3? Report your answer in scientific notation—and remember, you won't be reminded in future that all answers are to be reported with the correct significant digits. (b) How many electrons are in the cube?

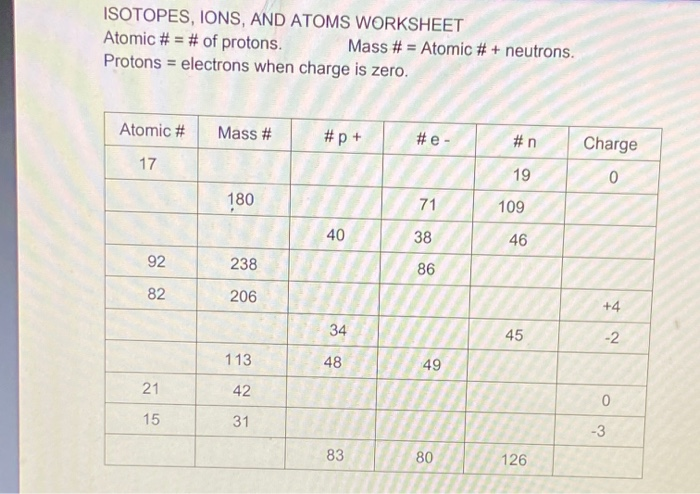
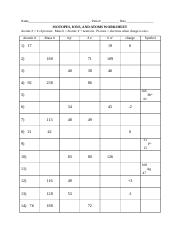
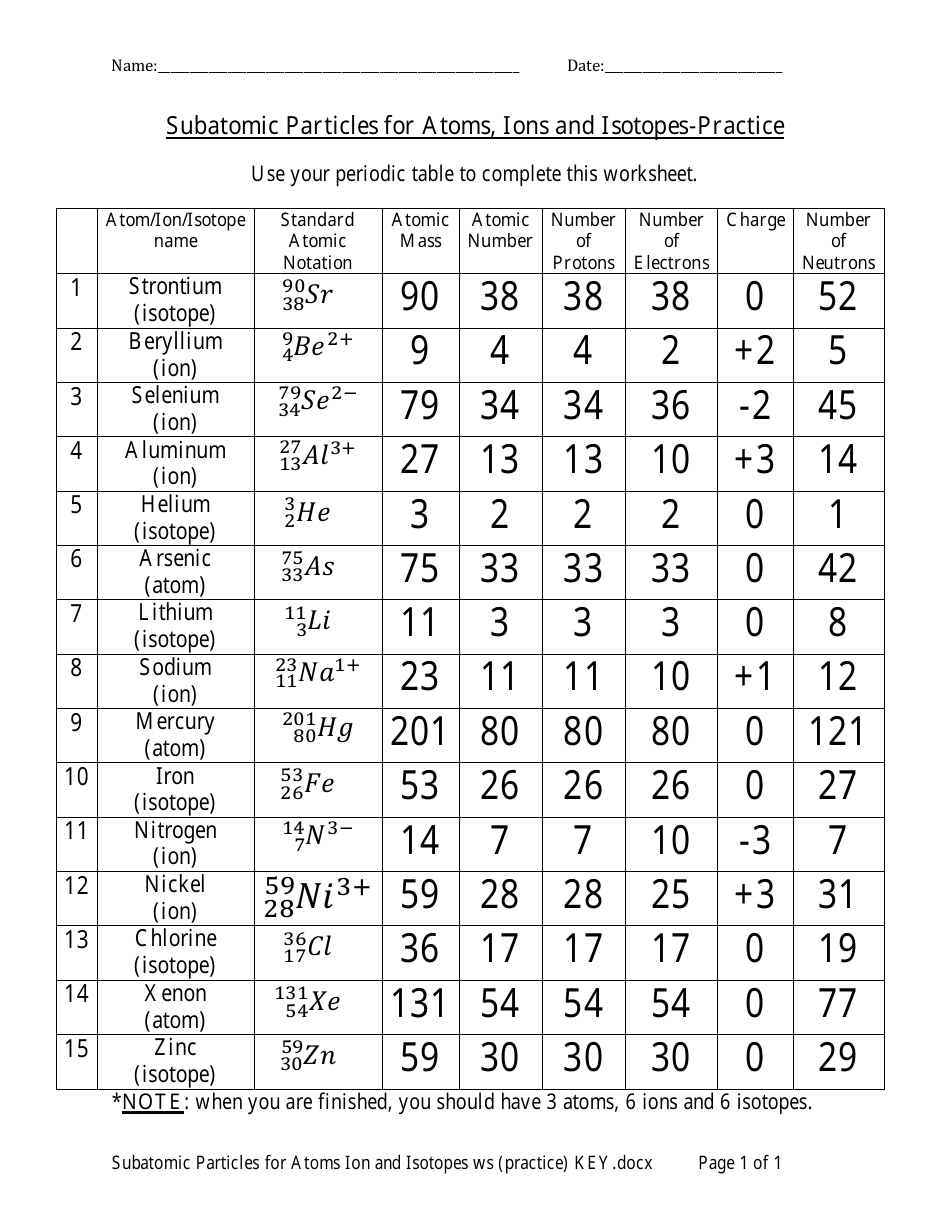
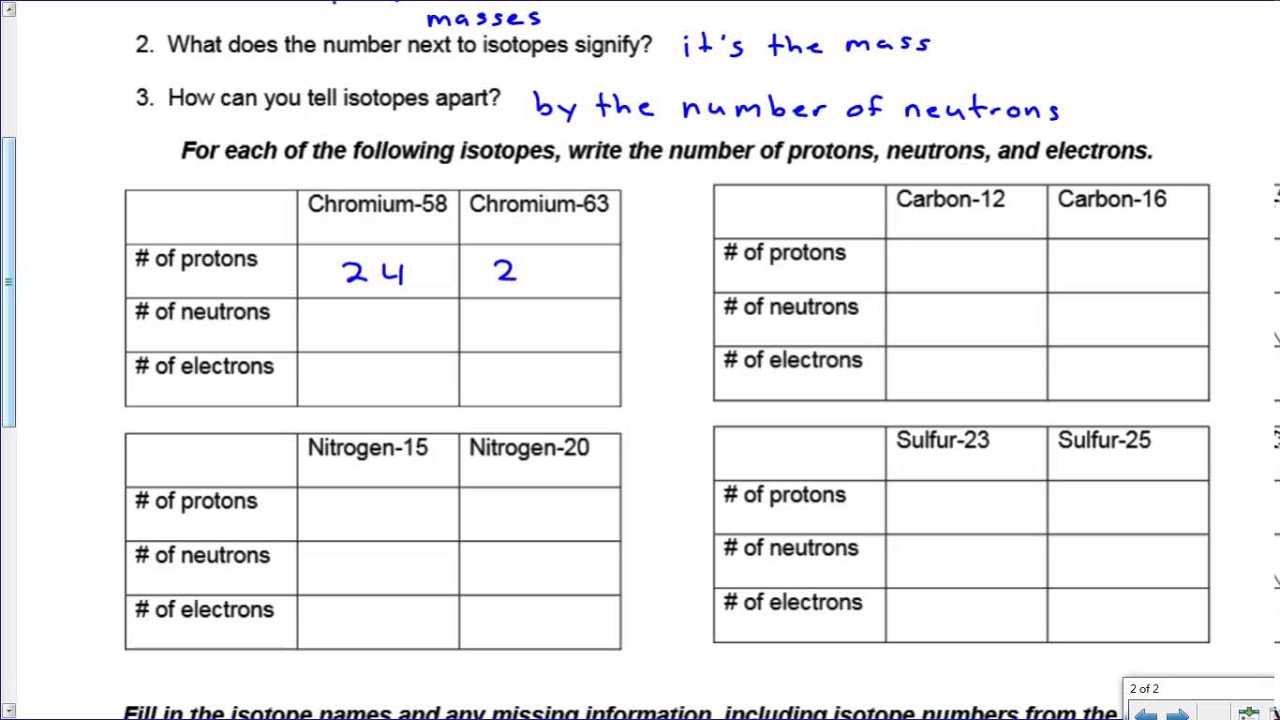
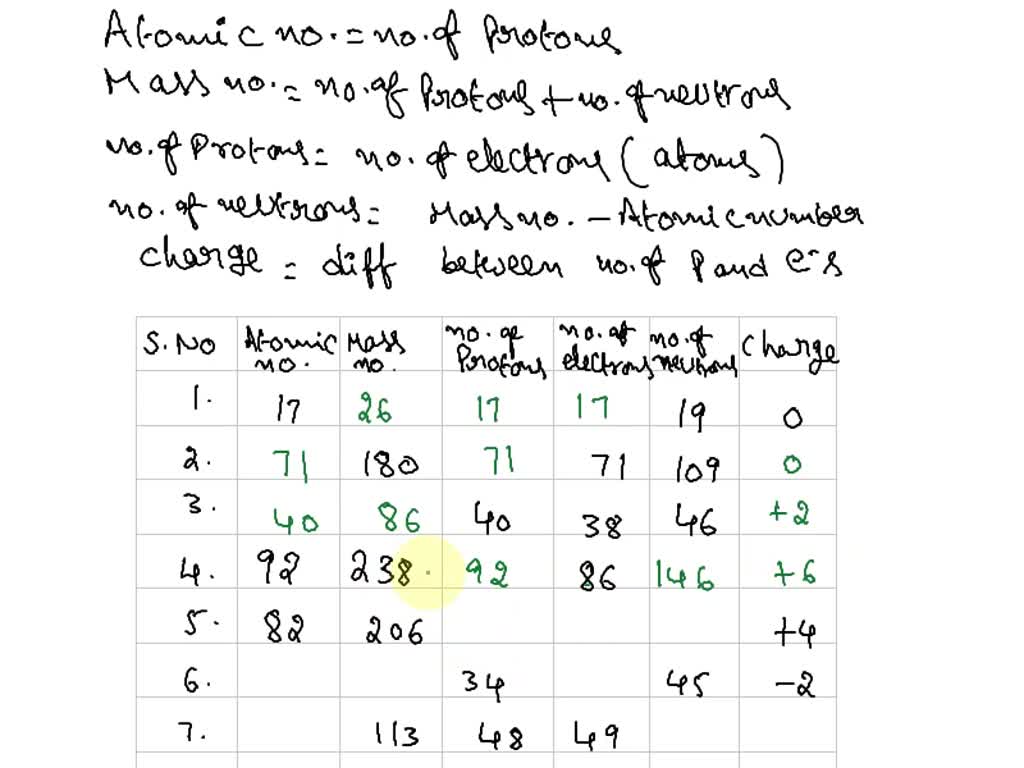
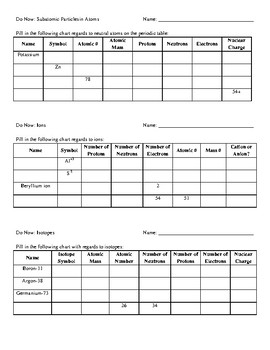
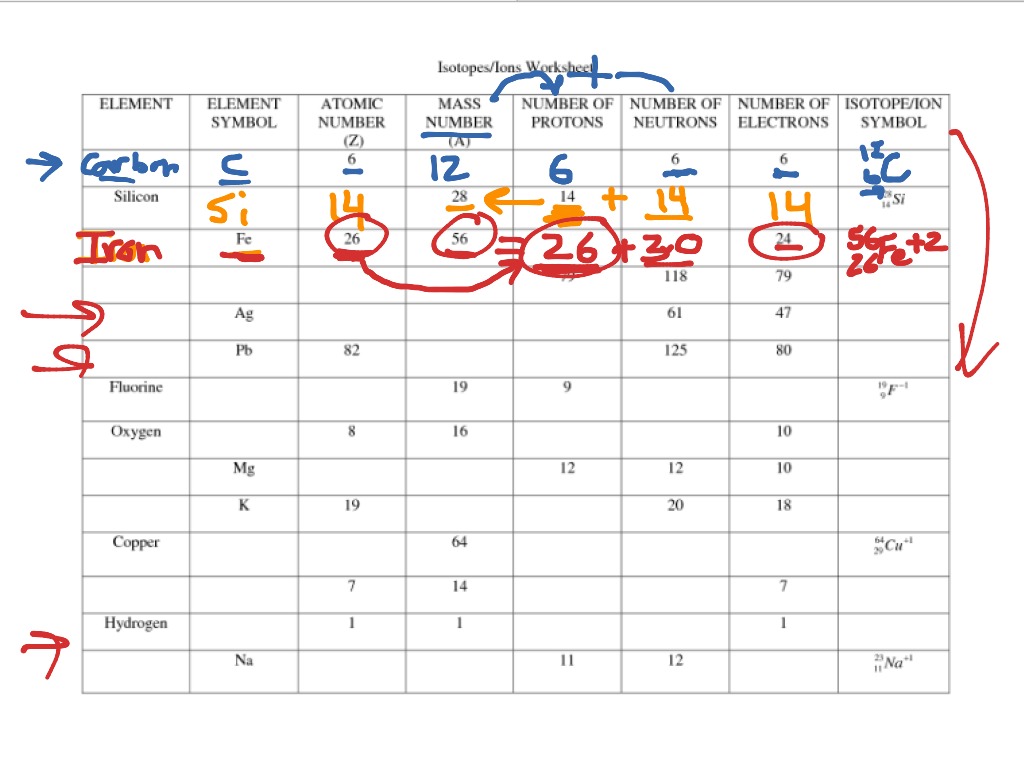
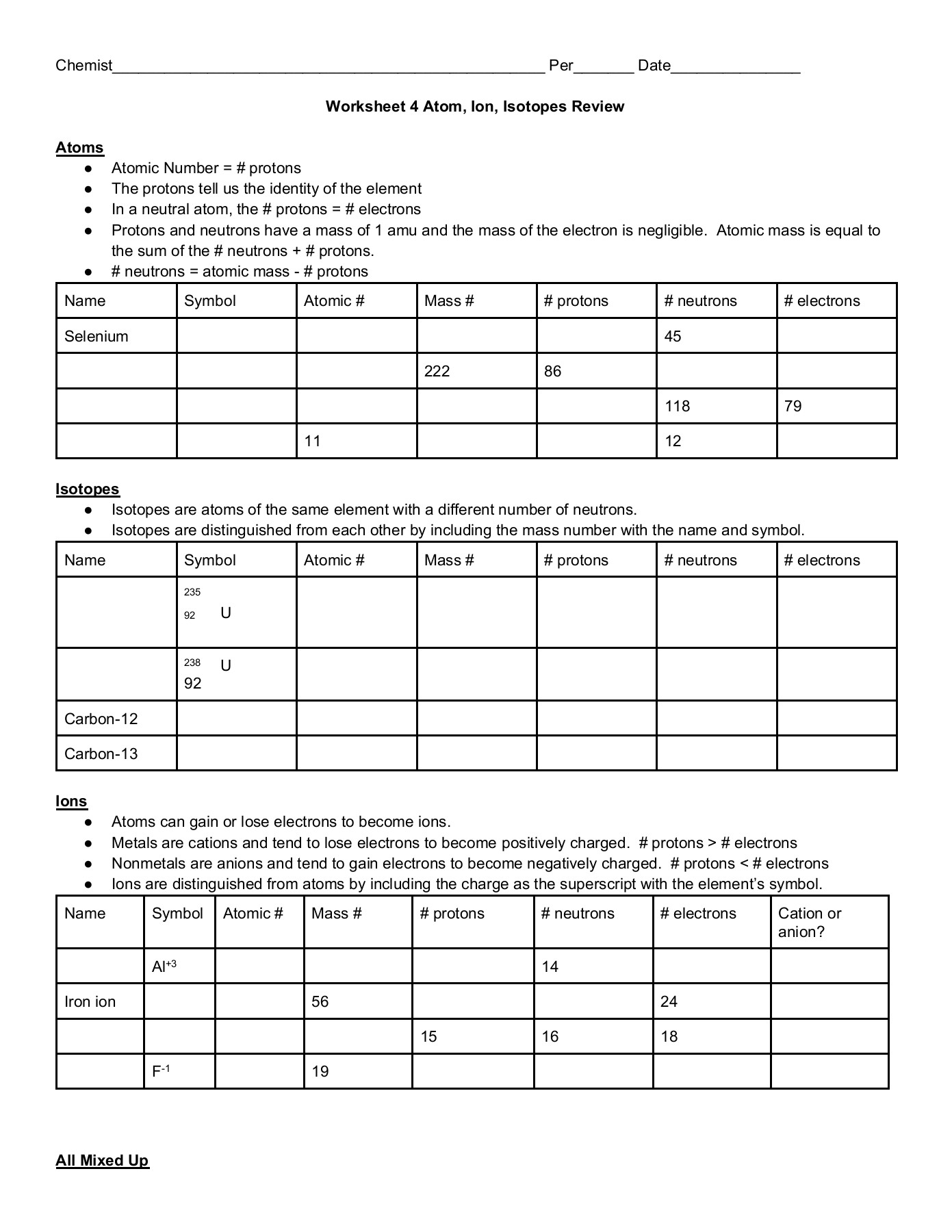
ISOTOPES, IONS, AND ATOMS WORKSHEET Atomic # = # of protons. Mass #= Atomic # Protons neutrons electrons when charge is zero., Atomic #, Mass #, #p, #e, Charge, 19, 180, 109, 40, 38, 46, 238, 86, 206, ...



















0 Response to "39 atoms ions isotopes worksheet answers"
Post a Comment