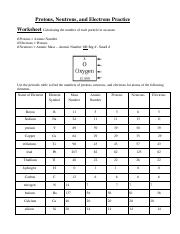
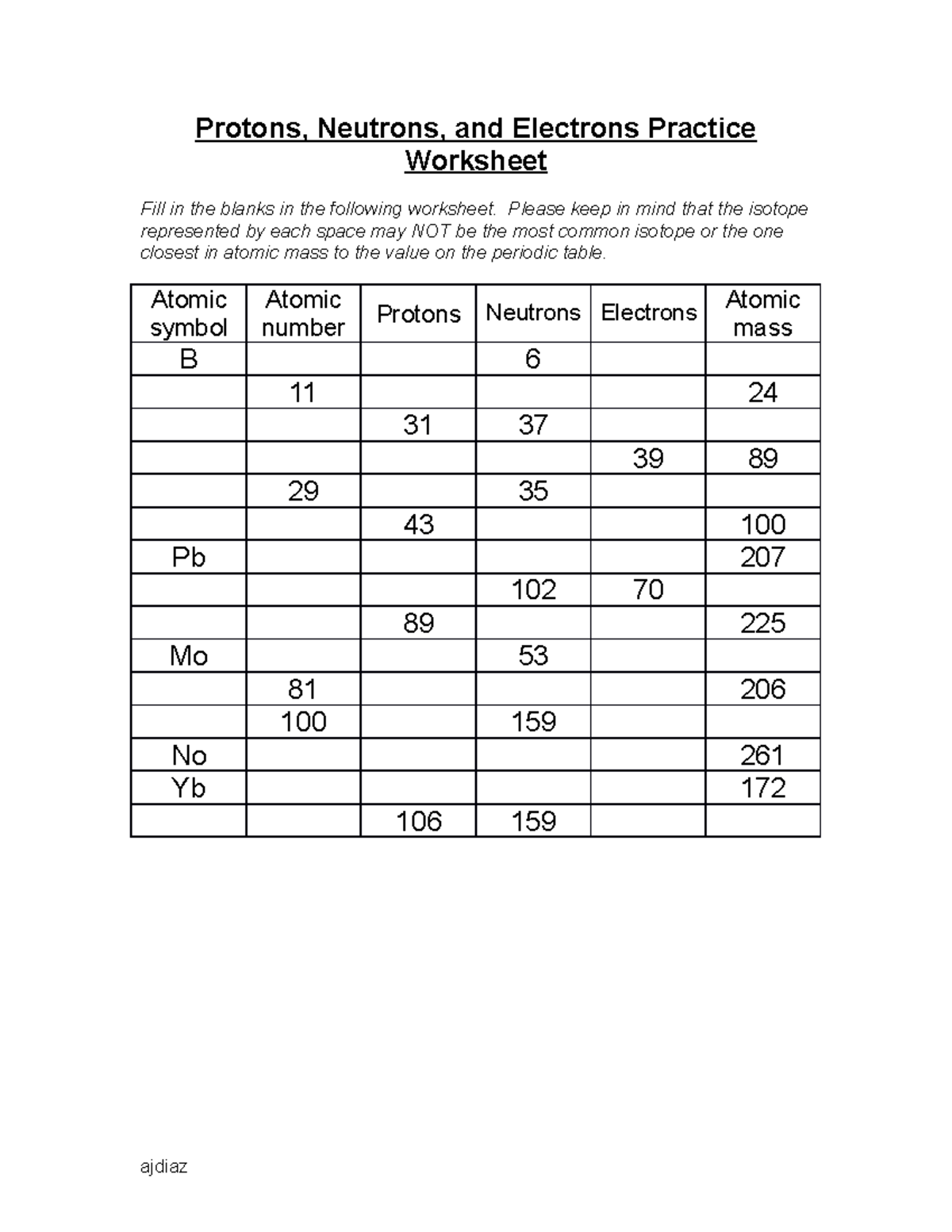
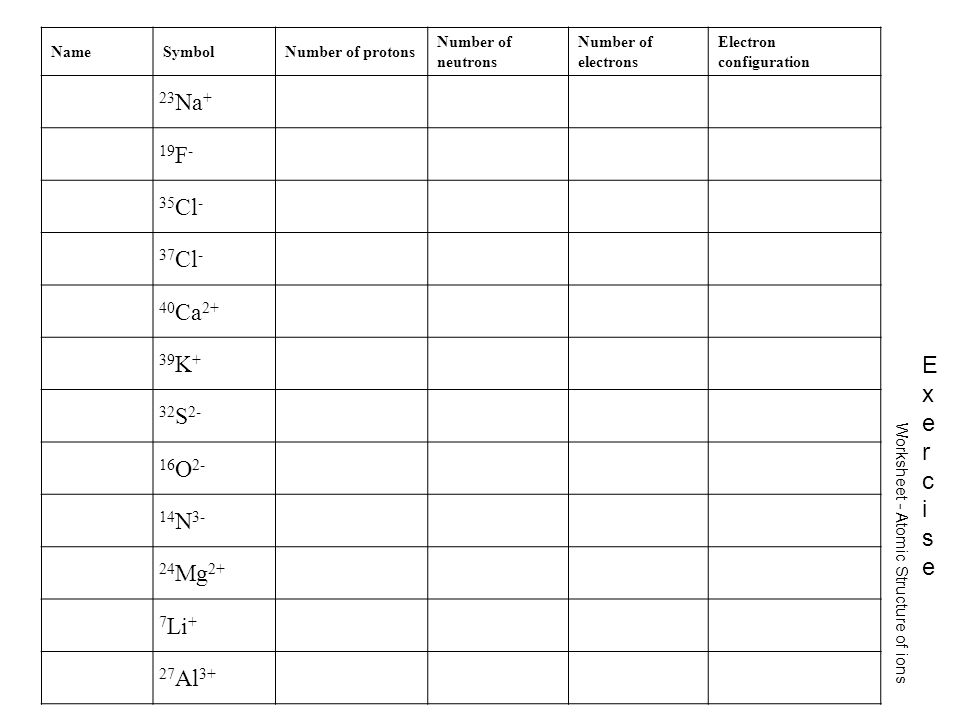
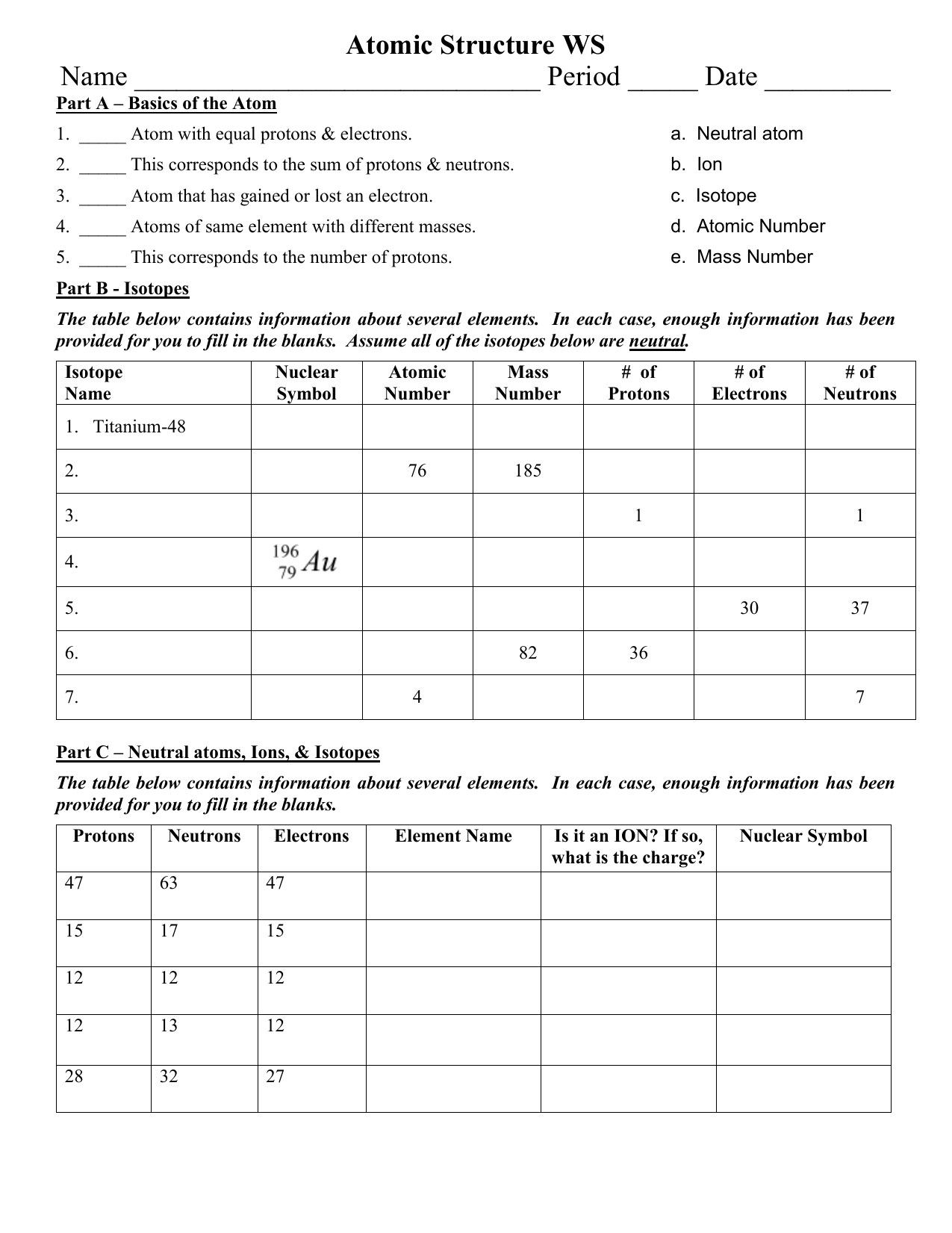
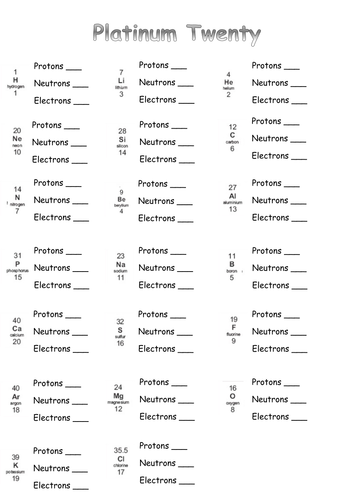
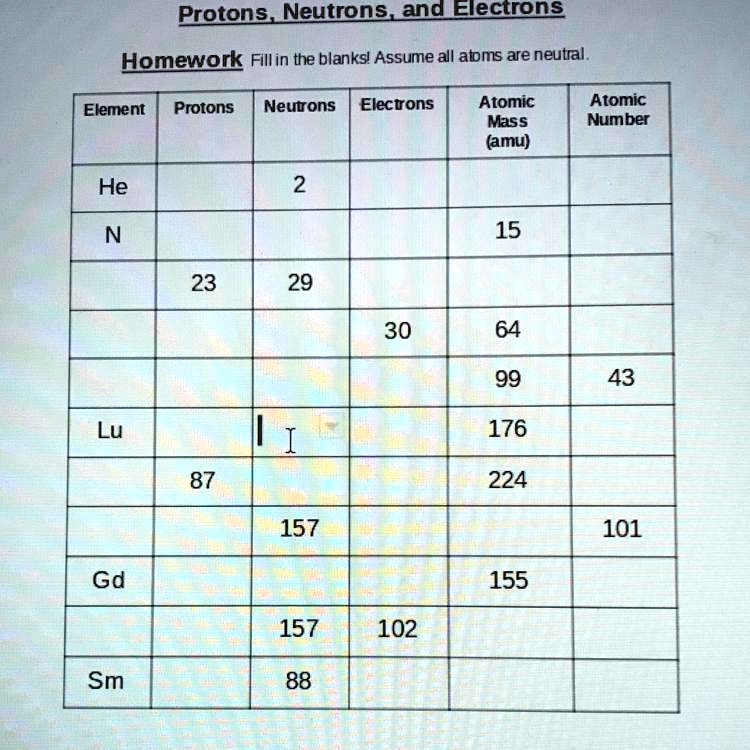
41 protons neutrons and electrons worksheet answers
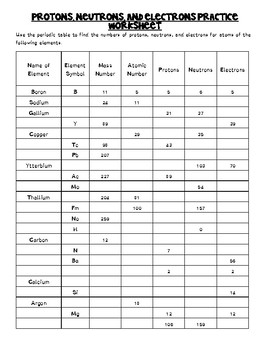
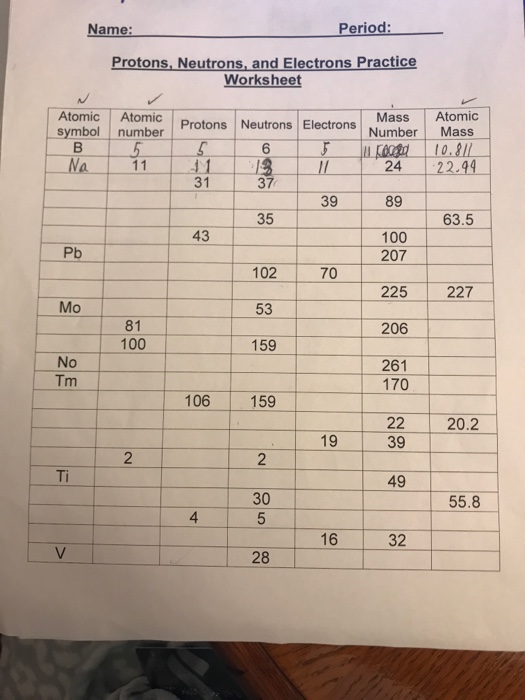
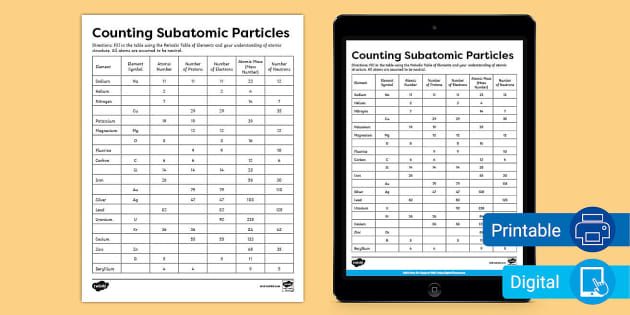
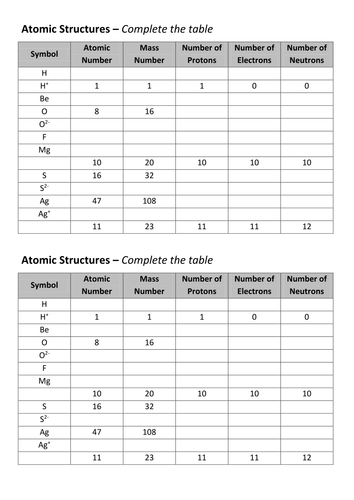
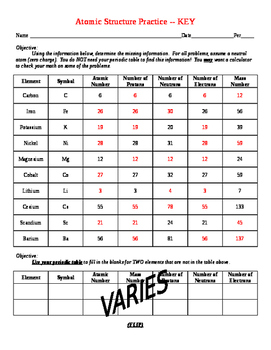
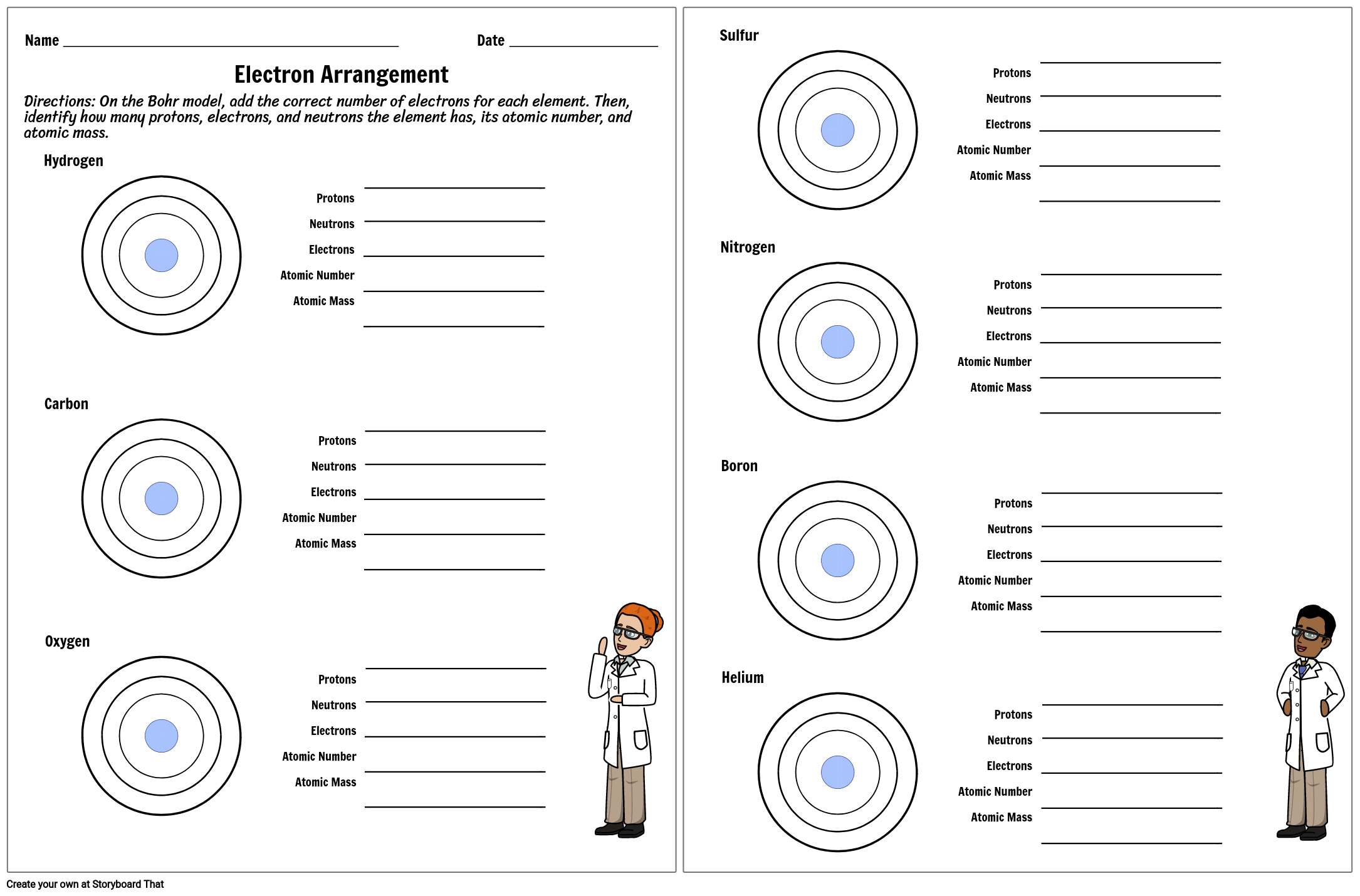
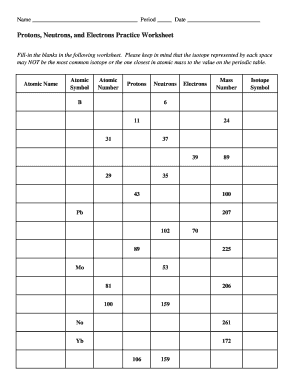
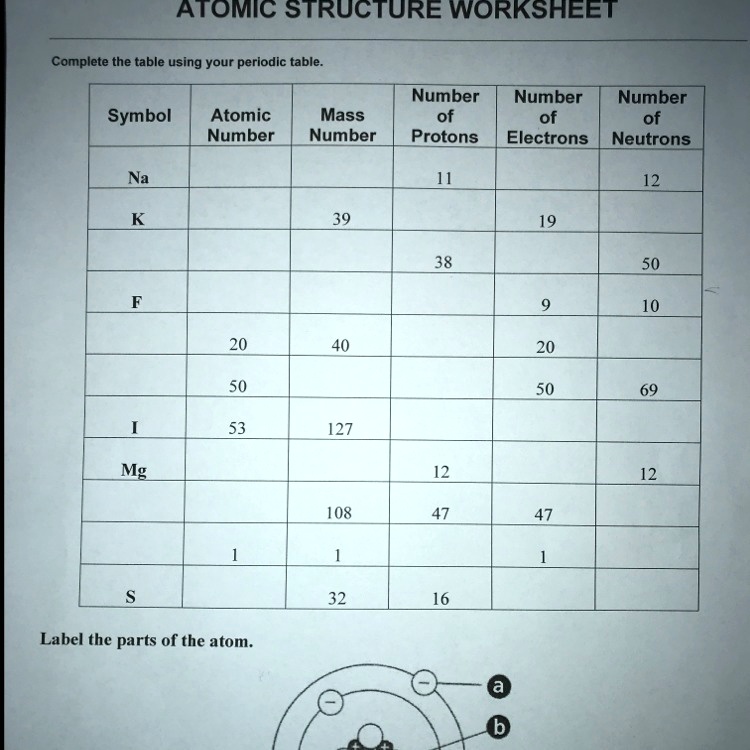
Atomic Mass and Atomic Number Worksheet Key Atomic Mass and Atomic Number Worksheet - Key Name of Element Symbol Atomic Number Atomic Mass Protons Neutrons Electrons copper Cu 29 64 29 35 29 tin Sn 50 119 50 69 50 iodine I 53 127 53 74 53 uranium U 92 238 92 146 92 potassium K 19 39 19 20 19 lithium Li 3 7 3 4 3 oxygen O 8 16 8 8 8 gold Au 79 197 79 118 79 ... Atomic Structure Worksheet - Washoe County School District Part l: Label the parts of this atom (nucleus, protons, electrons, neutrons) Part 2: Answer these: 1. The subatomic particle with no electrical charge is the 2. The subatomic particle with a positive charge is the 3. The subatomic particle with a negative charge is the 4. ... Atomic Structure Worksheet Author: Jennifer Brown Last modified by ...
Lewis Structure Worksheet #1 Read the Instructions for Drawing … Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 l Hydrogen 1 1 1 0 1 H Lithium 3 7 3 4 3 LiWebFile name : electron-dot-structures-lewis-structures-chemistry-worksheet-answers.pdf with Size pdf :11 megabytes.
Protons neutrons and electrons worksheet answers
Atomic number - Wikipedia The atomic number or nuclear charge number (symbol Z) of a chemical element is the charge number of an atomic nucleus.For ordinary nuclei, this is equal to the proton number (n p) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements.In an ordinary uncharged atom, the … Gumdrop Atoms - Activity - TeachEngineering Feb 25, 2020 · If there are more electrons than protons, the atom is negatively charged. (Note: these ions may or may not occur naturally.) The atom has 6 protons, 8 neutrons and 6 electrons. (Answer: The charge is neutral. The atom is carbon-14.) The atom has 11 protons, 11 neutrons and 10 electrons. (Answer: The charge is positive. (+1) The atom is sodium.) Home | ExploreLearning Solve the math fact fluency problem. Adaptive and individualized, Reflex is the most effective and fun system for mastering basic facts in addition, subtraction, multiplication and division for grades 2+.
Protons neutrons and electrons worksheet answers. Atomic Structure Worksheets - Easy Teacher Worksheets Atoms have three parts that work together. The protons carry a positive charge, while the electrons have a negative charge. The neutrons have no charge. All atoms have the same number of protons and electrons, and most atoms have the same number of protons and neutrons. The significance of atoms is that without them, nothing could exist. What is an Atom -Basics for Kids - YouTube Visit for more free science videos for kids.What is an Atom? A good video explaining atomic structure & molecules formation. An a... Chapter 4: The Periodic Table & Bonding - Middle School Chemistry The majority of the atomic mass is contributed by the protons and neutrons. For any element in the periodic table, the number of electrons in an atom of that element always equals the number of protons in the nucleus. But this is not true for neutrons. Atoms of the same element can have different numbers of neutrons than protons. Chemistry of Matter - Science Spot Protons Neutrons Electrons Li 3 4 3 P 15 16 15 Cl 17 18 17 Ni 59 28 28 K 19 19 20 Ag 108 47 47 H 1 0 1 Si 14 28 14 W 17 174 17 Ne 10 20 10 NOTE: The number protons and electrons is equal to the atomic number. To find neutrons, subtract the number of protons from the atomic mass. To find the atomic mass, add the number of protons and neutrons. ...
Home | ExploreLearning Solve the math fact fluency problem. Adaptive and individualized, Reflex is the most effective and fun system for mastering basic facts in addition, subtraction, multiplication and division for grades 2+. Gumdrop Atoms - Activity - TeachEngineering Feb 25, 2020 · If there are more electrons than protons, the atom is negatively charged. (Note: these ions may or may not occur naturally.) The atom has 6 protons, 8 neutrons and 6 electrons. (Answer: The charge is neutral. The atom is carbon-14.) The atom has 11 protons, 11 neutrons and 10 electrons. (Answer: The charge is positive. (+1) The atom is sodium.) Atomic number - Wikipedia The atomic number or nuclear charge number (symbol Z) of a chemical element is the charge number of an atomic nucleus.For ordinary nuclei, this is equal to the proton number (n p) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements.In an ordinary uncharged atom, the …






![Protons Neutrons And Electrons Practice Ws [6nq85590eznw]](https://idoc.pub/img/crop/300x300/6nq85590eznw.jpg)












0 Response to "41 protons neutrons and electrons worksheet answers"
Post a Comment