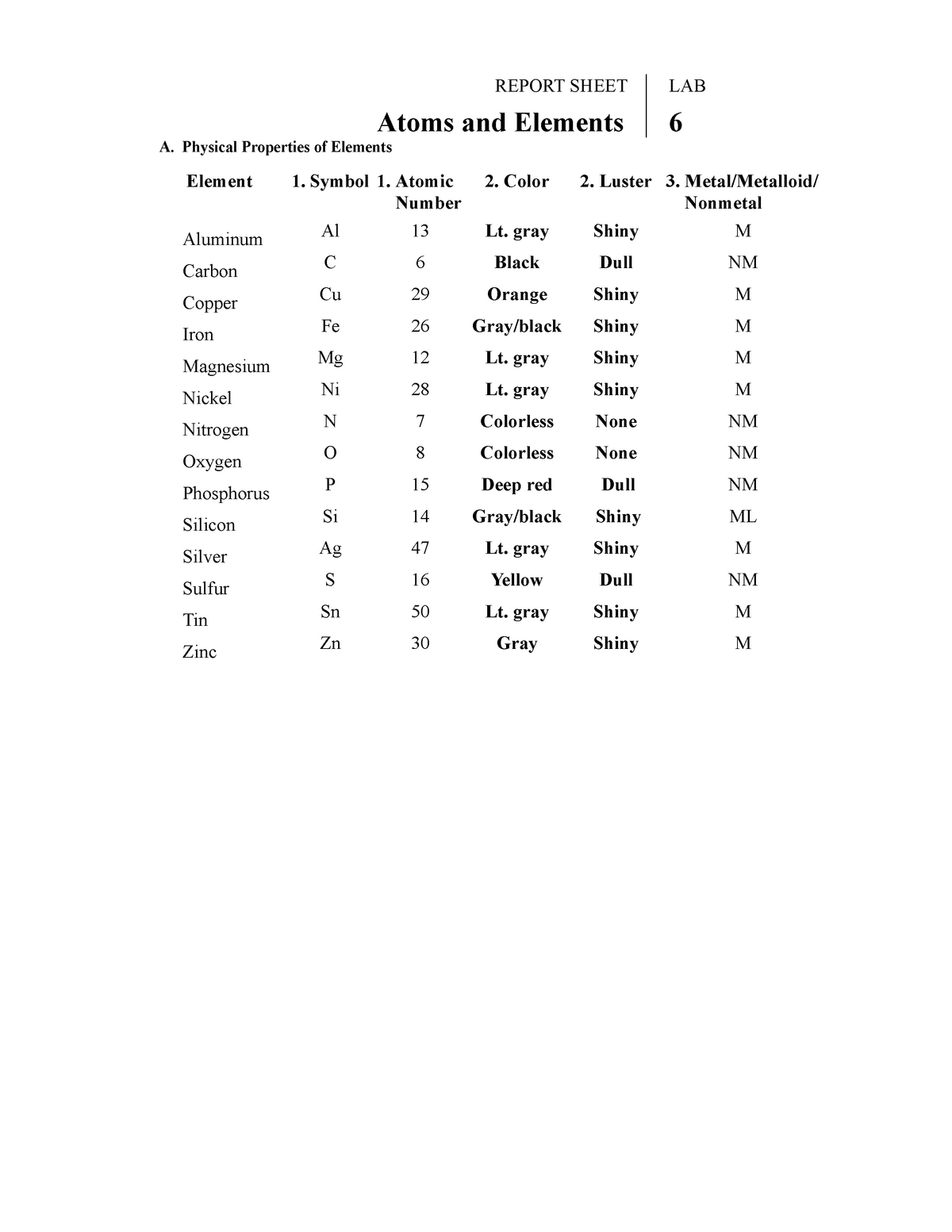
42 properties of atoms and the periodic table worksheet answers
Teaching resources | RSC Education Resources and materials to support your teaching of chemistry to primary, secondary and higher education students. This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities Chem4Kids.com: Elements & Periodic Table Up to this point in time, we have discovered or created about 120. Scientists just confirmed the creation of element 117 in 2014. While there are more elements to discover, the basic elements remain the same. Iron (Fe) atoms found on Earth are identical to iron atoms found on meteorites. The iron atoms in the red soil of Mars are also the same.
Free Printable Periodic Tables (PDF and PNG) Periodic Table of the Elements – 118 Elements IUPAC Standard Atomic Weights. This printable periodic table cites the IUPAC standard atomic mass values. This is an accurate up-to-date table for calculations and homework. Because only the borders of the element tiles are colored, the table is easy to read and kind to toner cartridges.

Properties of atoms and the periodic table worksheet answers
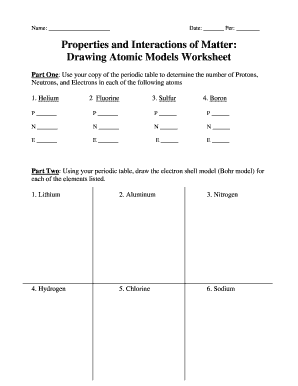
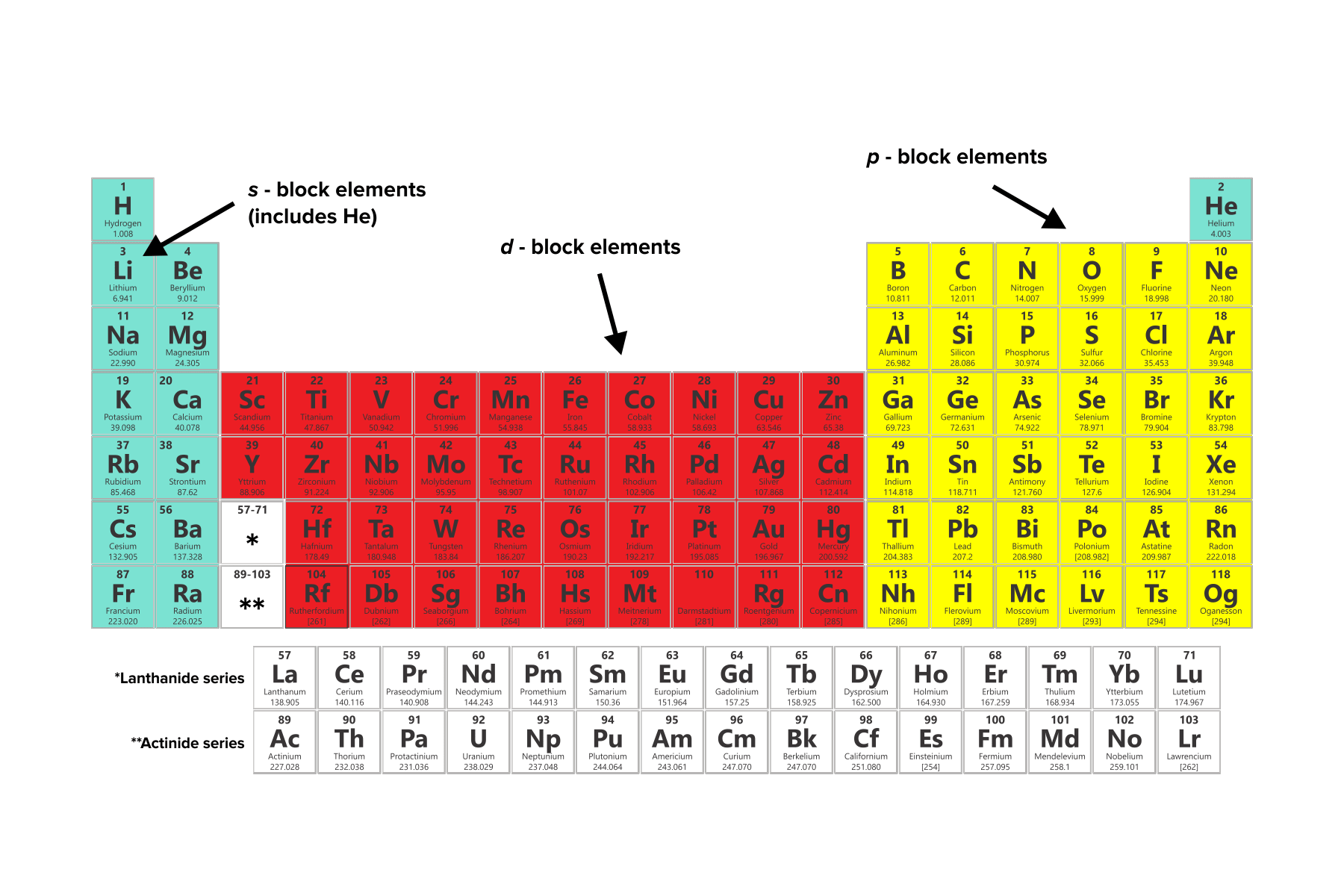
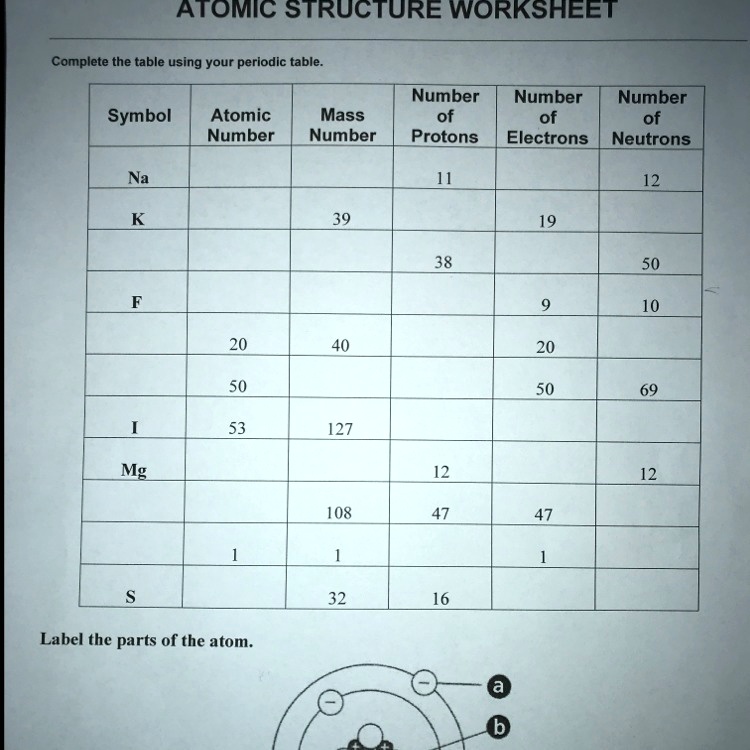
Atomic Structure Worksheets - Easy Teacher Worksheets Why Atoms Have Atomic Numbers on the Periodic Table. The atoms in each unique element have a specific number of protons. For example, oxygen has two atoms so its atomic number is 2. Iron has twenty-six protons in its nucleus so the atomic number is 26. Scientists can identify an element by its atomic number on the chart. Conclusion 2.3: Families and Periods of the Periodic Table Periods of the Periodic Table. If you can locate an element on the Periodic Table, you can use the element's position to figure out the energy level of the element's valence electrons. A period is a horizontal row of elements on the periodic table. For example, the elements sodium (\(\ce{Na}\)) and magnesium (\(\ce{Mg}\)) are both in period 3. Electrolysis of molten lead(II) bromide - RSC Education 1. Periodic table and atomic structure. 1.5 Oxidation and Reduction. Depth of Treatment. Introduction to oxidation and reduction: simple examples only, e.g. Na with Cl₂, Mg with O₂, Zn with Cu²⁺. Oxidation and reduction in terms of loss and gain of electrons.
Properties of atoms and the periodic table worksheet answers. Atomic number - Wikipedia Loosely speaking, the existence or construction of a periodic table of elements creates an ordering of the elements, and so they can be numbered in order.. Dmitri Mendeleev claimed that he arranged his first periodic tables (first published on March 6, 1869) in order of atomic weight ("Atomgewicht"). However, in consideration of the elements' observed chemical properties, he … 7.4 Formal Charges and Resonance - Chemistry 2e | OpenStax All oxygen atoms, however, are equivalent, and the double bond could form from any one of the three atoms. This gives rise to three resonance forms of the carbonate ion. Because we can write three identical resonance structures, we know that the actual arrangement of electrons in the carbonate ion is the average of the three structures. Molecular Formula Practice Test Questions - ThoughtCo Aug 01, 2019 · The molecular formula of a compound is a representation of the number and type of elements present in one molecular unit of the compound. This 10-question practice test deals with finding the molecular formula of chemical compounds.. A periodic table will be required to complete this test. Answers appear after the final question. Oxygen - Element information, properties and uses | Periodic Table Uses and properties John Emsley, Nature’s Building Blocks: An A-Z Guide to the Elements, Oxford University Press, New York, 2nd Edition, 2011. Thomas Jefferson National Accelerator Facility - Office of Science Education, It’s Elemental - The Periodic Table of Elements, accessed December 2014. Periodic Table of Videos, accessed December 2014.
Electrolysis of molten lead(II) bromide - RSC Education 1. Periodic table and atomic structure. 1.5 Oxidation and Reduction. Depth of Treatment. Introduction to oxidation and reduction: simple examples only, e.g. Na with Cl₂, Mg with O₂, Zn with Cu²⁺. Oxidation and reduction in terms of loss and gain of electrons. 2.3: Families and Periods of the Periodic Table Periods of the Periodic Table. If you can locate an element on the Periodic Table, you can use the element's position to figure out the energy level of the element's valence electrons. A period is a horizontal row of elements on the periodic table. For example, the elements sodium (\(\ce{Na}\)) and magnesium (\(\ce{Mg}\)) are both in period 3. Atomic Structure Worksheets - Easy Teacher Worksheets Why Atoms Have Atomic Numbers on the Periodic Table. The atoms in each unique element have a specific number of protons. For example, oxygen has two atoms so its atomic number is 2. Iron has twenty-six protons in its nucleus so the atomic number is 26. Scientists can identify an element by its atomic number on the chart. Conclusion

































0 Response to "42 properties of atoms and the periodic table worksheet answers"
Post a Comment