40 section 10.3 percent composition and chemical formulas worksheet answers
Chapter10 section03 Percent Composition and Chemical Formulas By Hamd… Chapter10 section03 Percent Composition and Chemical Formulas By Hamdy Karim. May. 21, 2014 • 3 likes • 1,712 views Download Now Download to read offline Education Technology Students will learn about the Percent Composition and Chemical Formulas, also they will learn the difference between the empirical and molecular formulae! Hamdy Karim Follow empirical formula and molecular formula worksheet answers worksheet formula jul. Empirical And Molecular Formula Sheet Worksheet For 10th - Higher Ed . empirical molecular. Empirical And Molecular Formula Worksheet Answer Key formulae2020jakarta.blogspot.com. empirical atoms counting. 36 Section 10.3 Percent Composition And Chemical Formulas Worksheet chripchirp.blogspot.com
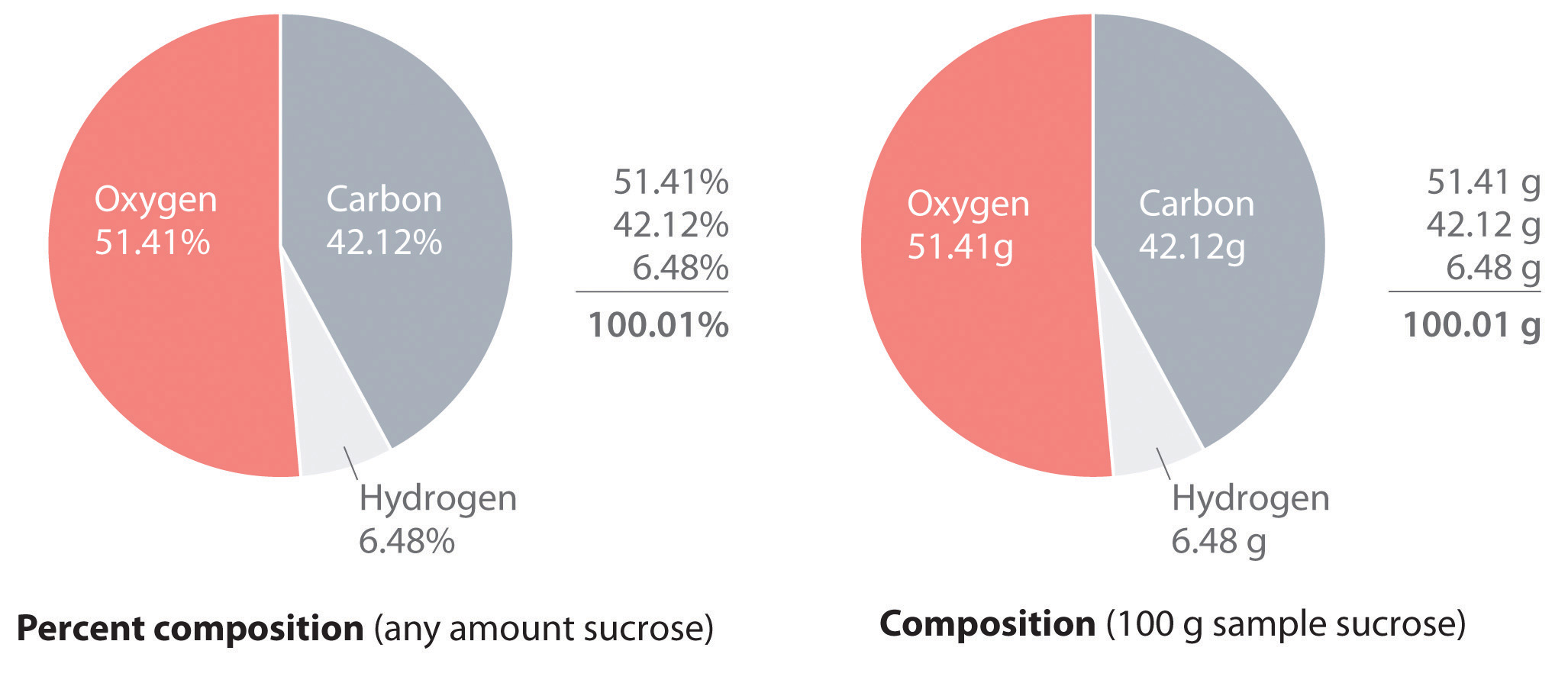
PDF 10.3 Percent Composition and Chemical Formulas 10 the percent compositionor the percent by mass of each element in the compound. The percent composition of a compound consists of a percent value for each different element in the compound. As you can see in Figure 10.13, the percent composition of K 2 CrO 4 is K 40.3%, Cr 26.8%, and O 32.9%. These percents must total 100% (40.3% 26.8% 32.9% 100%).

Section 10.3 percent composition and chemical formulas worksheet answers
10.3 percent composition & chemical formulas answer key/answers Name Date Section Revgew Objectives $ .' PI!It ., Class PERCENT COMPOSITION . , , , , AND Part 0 Problems Solve the fallowing problems in the space provided. Shaw your… 10.3 Percent Composition & Chemical Formulas Answer Key/answers If 3.907 g of carbon combines completely with 0.874 g of hydrogen compound, what is the percent composition of this compound? 4. Frorn the formula for culciurn acetate, Ca(C,H30,)" calculate carbon that can be obtained frorn 65.3 g 01" the compound. Chemistry (12th Edition) Chapter 10 - Chemical Quantities - 10.3 ... Chapter 10 - Chemical Quantities - 10.3 Percent Composition and Chemical Formulas - 10.3 Lesson Check - Page 333: 45 Answer The molecular formula of a compound is either the same as its experimentally determined empirical formula, or it is a simple whole-number multiple of its empirical formula. Work Step by Step
Section 10.3 percent composition and chemical formulas worksheet answers. 103 Percent Composition And Chemical Formulas Answer Key 103 Percent Composition And Chemical Formulas Answer Key 6.7: Mass Percent Composition from a Chemical Formula he percent composition of a compound can also be determined from the formula of the compound. The subscripts in the formula are first used to calculate the mass of each element in one mole of the compound. Chemistry (12th Edition) Chapter 10 - Chemical Quantities - 10.3 ... Chapter 10 - Chemical Quantities - 10.3 Percent Composition and Chemical Formulas - 10.3 Lesson Check - Page 333: 48 Answer 313.6 Work Step by Step H= 124 g/mol Ca (CHO) = 158.1658 H= 124 x 4 = 496 496/158.1658 x100 =313.6 Update this answer! You can help us out by revising, improving and updating this answer. Update this answer PPT Section 10.3 Percent Composition and Chemical Formulas Calculating Percent Composition of a Compound Like all percent problems: part whole Find the mass of each of the components (the elements), Next, divide by the total mass of the compound; then x 100 x 100 % = percent Example Calculate the percent composition of a compound that is made of 29.0 grams of Ag with 4.30 grams of S. 29.0 g Ag 33.3 g ... 10.3 Percent Composition & Chemical Formulas Answer Key/Answers Section 10.3 X 284 g Mn2P :P7 201 a Hg c. b X 100 233 g HgS 32.1 S X 100 233 g hg 100 = 39.4% 0 = 1. Percent Percent Percent 5.34 = a b 86.3% Ha
10.3 Percent Composition and Chemical Formulas - Pittsfield Percent Composition of a Compound K = 40.3% Cr = 26.8% + O = 32.9% 100% These percents must total 100%. 10.3 Percent Composition and Chemical Formulas 7 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. Percent Composition of a Compound These percents must total 100%. • The percent composition of a compound is 10.3-Percent Composition and Chemical Formulas - Quizlet 10.3-Percent Composition and Chemical Formulas STUDY Flashcards Learn Write Spell Test PLAY Match Gravity Created by eaecco Terms in this set (8) The percent by mass of an element in a compound is the number of grams of the element divided by the mass in grams of the compound, multiplied by 100% TO KNOW Percent composition 10.3 percent composition & chemical formulas answer key/answers Name Date Section Revgew Objectives $ .' PI!It ., Class PERCENT COMPOSITION . , , , , AND Part 0 Problems Solve the fallowing problems in the space provided. ... 10.3 percent composition & chemical formulas answer key/answers. Home; Documents; 10.3 Percent Composition & Chemical Formulas Answer Key/Answers; Match case Limit results 1 per page ... Chapter 10.3 Percent Composition and Chemical Formulas The percent by mass of an element in a compound is the number of grams of the element divided by the mass in grams of the compound, multiplied by 100%. empirical formula a formula with the lowest whole-number ratio of elements in a compound; the empirical formula of hydrogen peroxide (H2O2) is HO The empirical formula of a compound shows
Chemistry (12th Edition) Chapter 10 - Chemical Quantities - 10.3 ... Chapter 10 - Chemical Quantities - 10.3 Percent Composition and Chemical Formulas - 10.3 Lesson Check - Page 333: 45 Answer The molecular formula of a compound is either the same as its experimentally determined empirical formula, or it is a simple whole-number multiple of its empirical formula. Work Step by Step 10.3 Percent Composition & Chemical Formulas Answer Key/answers If 3.907 g of carbon combines completely with 0.874 g of hydrogen compound, what is the percent composition of this compound? 4. Frorn the formula for culciurn acetate, Ca(C,H30,)" calculate carbon that can be obtained frorn 65.3 g 01" the compound. 10.3 percent composition & chemical formulas answer key/answers Name Date Section Revgew Objectives $ .' PI!It ., Class PERCENT COMPOSITION . , , , , AND Part 0 Problems Solve the fallowing problems in the space provided. Shaw your…


























0 Response to "40 section 10.3 percent composition and chemical formulas worksheet answers"
Post a Comment