40 chemistry atomic number and mass number worksheet answers
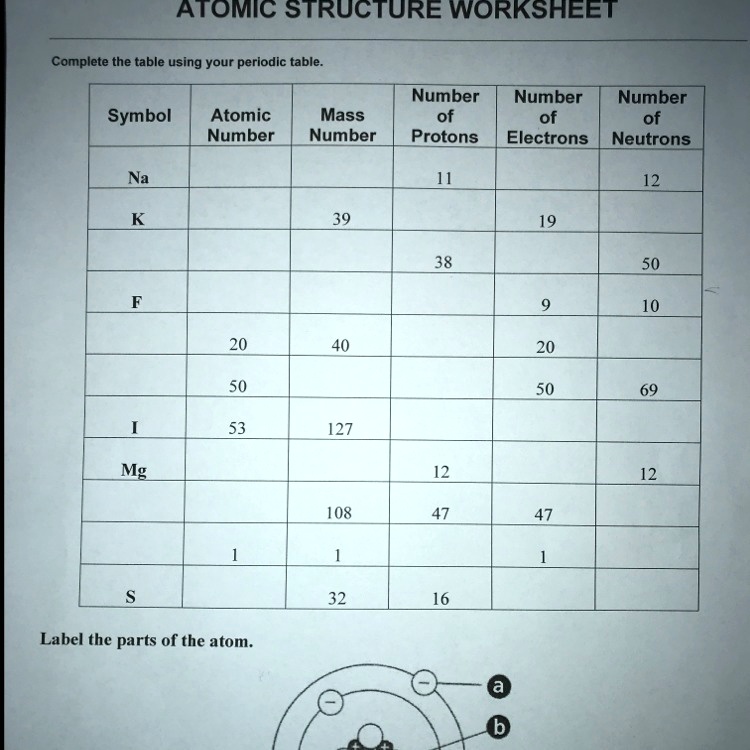
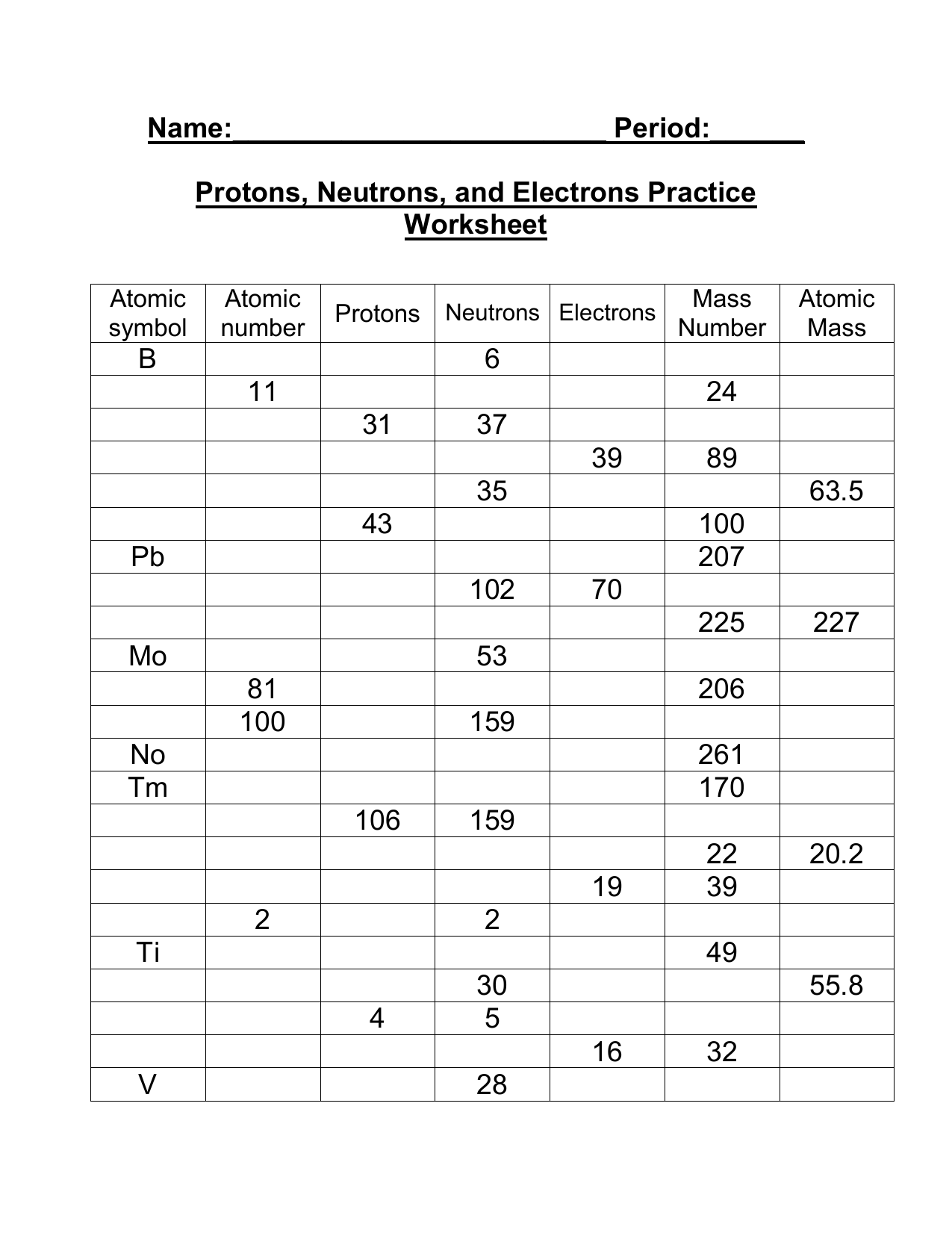
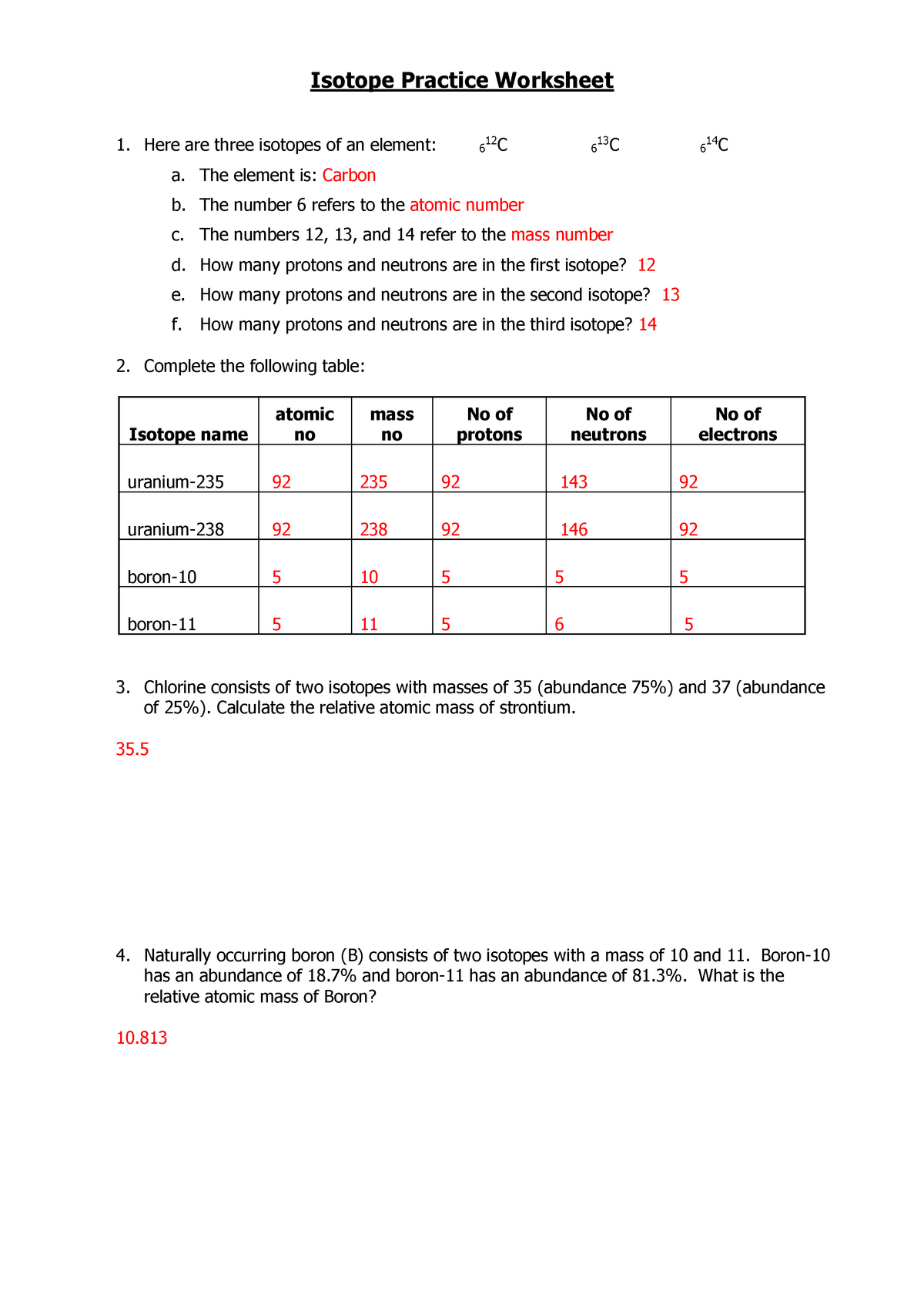
› cms › lib07Atomic Mass and Atomic Number Worksheet Key Atomic Mass and Atomic Number Worksheet - Key Name of Element Symbol Atomic Number Atomic Mass Protons Neutrons Electrons copper Cu 29 64 29 35 29 tin Sn 50 119 50 69 50 iodine I 53 127 53 74 53 uranium U 92 238 92 146 92 potassium K 19 39 19 20 19 lithium Li 3 7 3 4 3 oxygen O 8 16 8 8 8 gold Au 79 197 79 118 79 en.wikipedia.org › wiki › ChemistryChemistry - Wikipedia The mass number is the sum of the number of protons and neutrons in a nucleus. Although all the nuclei of all atoms belonging to one element will have the same atomic number, they may not necessarily have the same mass number; atoms of an element which have different mass numbers are known as isotopes .
› cms › lib6Basic Atomic Structure Worksheet Key - Neshaminy School District have the atomic number. The mQSS of an element is the average mass of an element's naturally occurring atom, or isotopes, taking into account the Of each isotope. The q of an element is the total number of protons and neutrons in the of atom. The mass number is used to calculate the number of O in one atom of an element. In
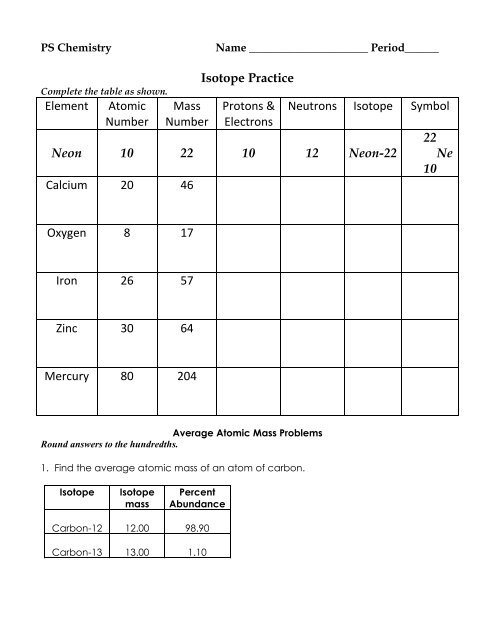
Chemistry atomic number and mass number worksheet answers
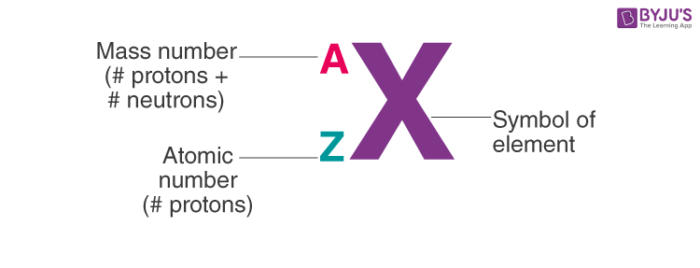
wou.edu › chemistry › coursesChapter 6 – Quantities in Chemical Reactions – Chemistry For a larger molecule, like glucose (C 6 H 12 O 6), that has multiple atoms of the same type, simply multiply the atomic mass of each atom by the number of atoms present, and then add up all the atomic masses to get the final molecular mass. The mole concept can be extended to masses of formula units and molecules as well. › cms › lib8Metric System Handout/Worksheet Integrated Science 1 Redwood ... tells you the number of _____ in a neutral atom of that element. The atomic number gives the “identity” of an element as well as its location on the periodic table. No two different elements will have the _____ atomic number. 4. en.wikipedia.org › wiki › Atomic_numberAtomic number - Wikipedia The atomic number or nuclear charge number (symbol Z) of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (n p) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements.
Chemistry atomic number and mass number worksheet answers. study.com › academy › practiceQuiz & Worksheet - Atomic Number and Mass Number | Study.com Print Atomic Number and Mass Number Worksheet 1. If Atom #1 has 19 protons and 22 neutrons, and Atom #2 has 20 protons and 22 neutrons, are these isotopes of the same element? en.wikipedia.org › wiki › Atomic_numberAtomic number - Wikipedia The atomic number or nuclear charge number (symbol Z) of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (n p) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements. › cms › lib8Metric System Handout/Worksheet Integrated Science 1 Redwood ... tells you the number of _____ in a neutral atom of that element. The atomic number gives the “identity” of an element as well as its location on the periodic table. No two different elements will have the _____ atomic number. 4. wou.edu › chemistry › coursesChapter 6 – Quantities in Chemical Reactions – Chemistry For a larger molecule, like glucose (C 6 H 12 O 6), that has multiple atoms of the same type, simply multiply the atomic mass of each atom by the number of atoms present, and then add up all the atomic masses to get the final molecular mass. The mole concept can be extended to masses of formula units and molecules as well.



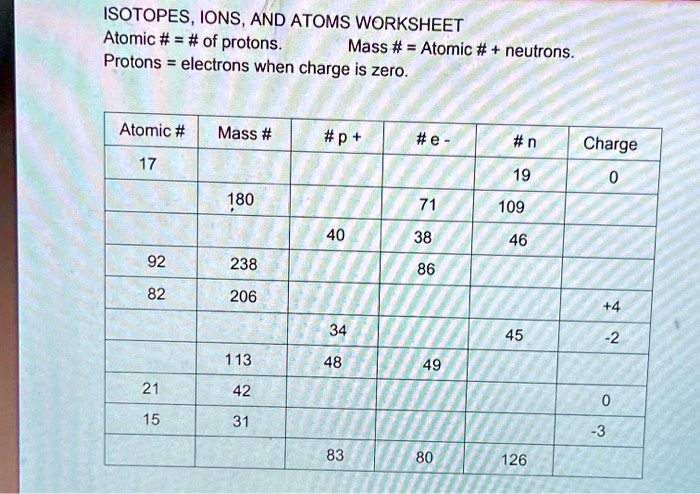
:max_bytes(150000):strip_icc()/PeriodicTableMuted-56a12d823df78cf772682aaa.png)

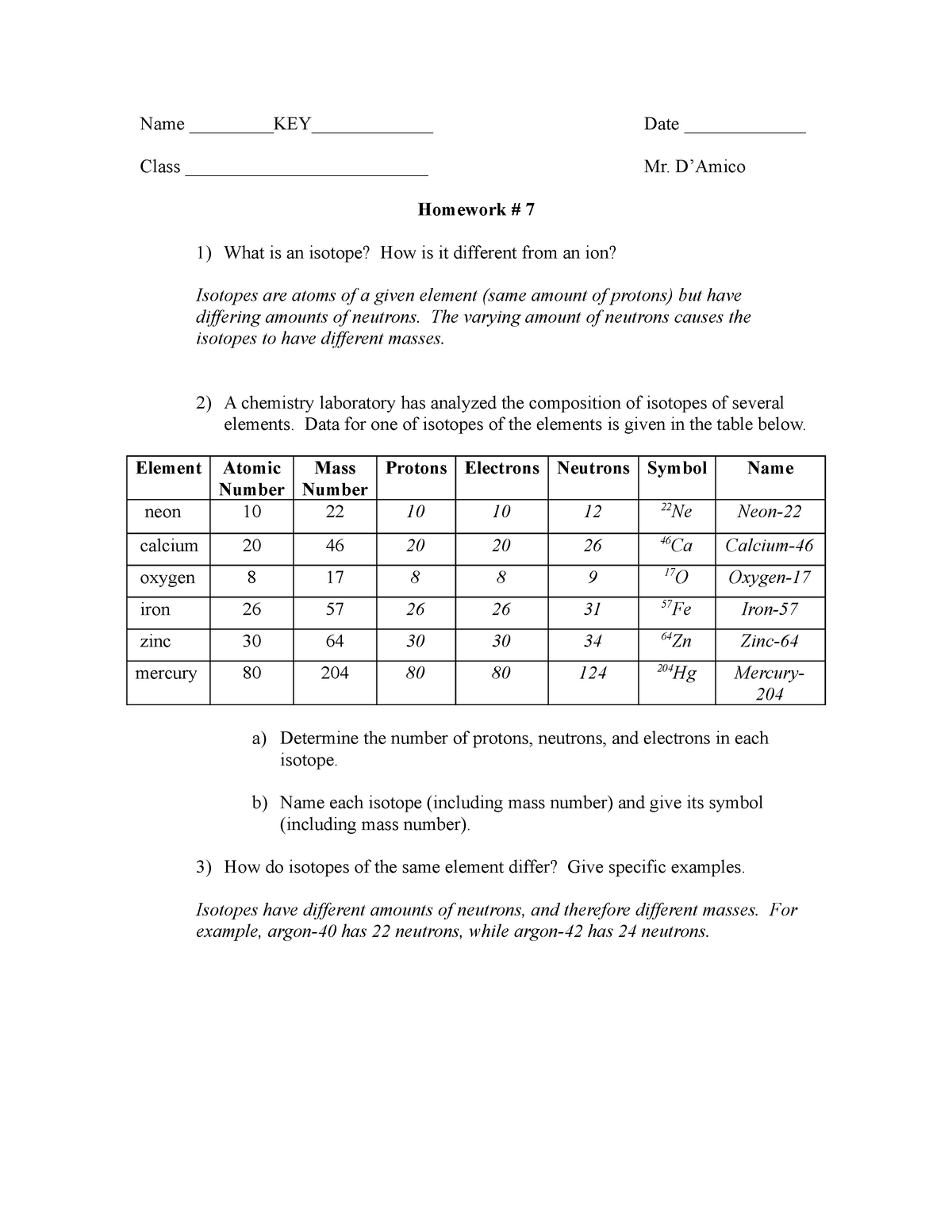










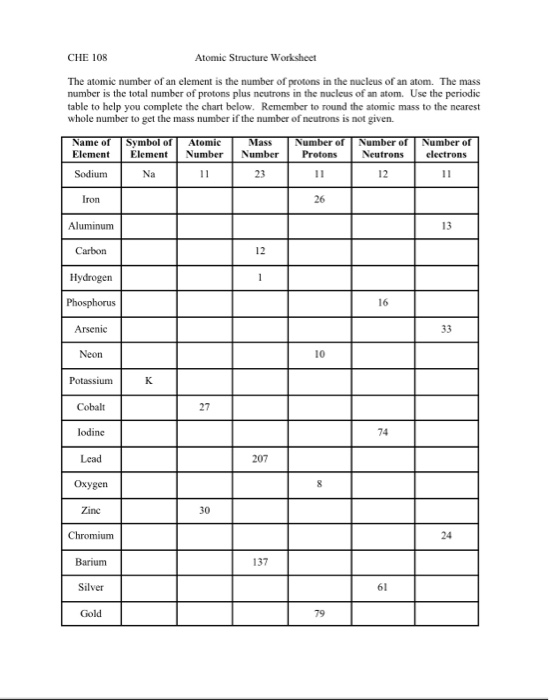




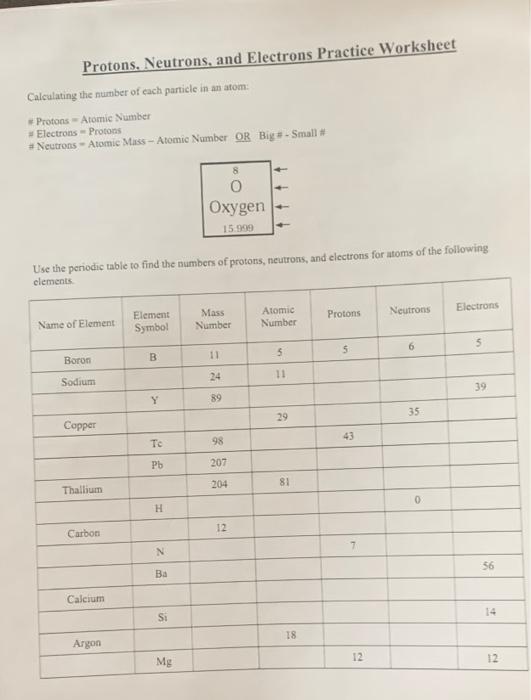


/element-list-names-atomic-numbers-606529_V1_FINAL-f332cfc84a494b7782d84fc986cdaf86.png)
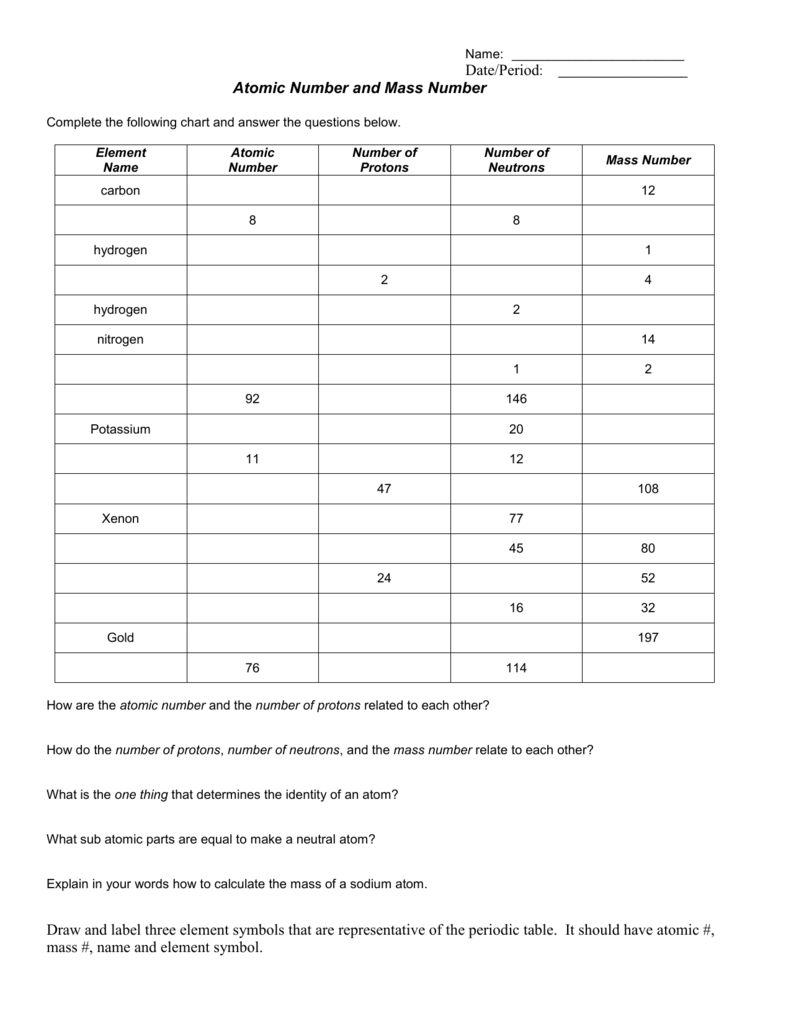
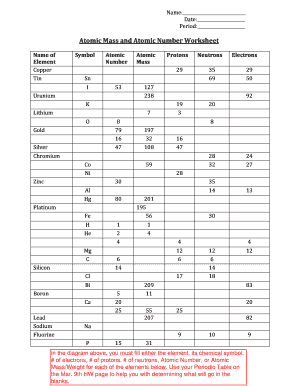

0 Response to "40 chemistry atomic number and mass number worksheet answers"
Post a Comment